Top 50 Pharmaceutical Companies R&D Progress: An Overview of Ipsen's 126 Drug Pipelines
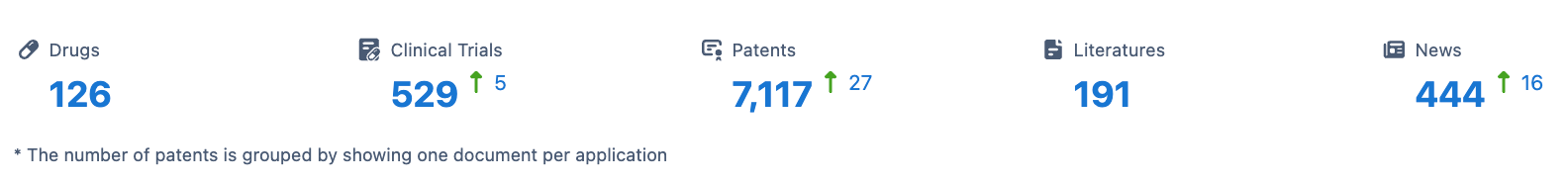
Ipsen SA is a pharmaceutical organization that was founded in 1929 and is based in France. The company operates in the field of biomedicine and has a diverse portfolio of drugs targeting various therapeutic areas. In this report, we will analyze the distribution of therapeutic areas, the most frequently developed targets, and the pipeline of Ipsen SA.
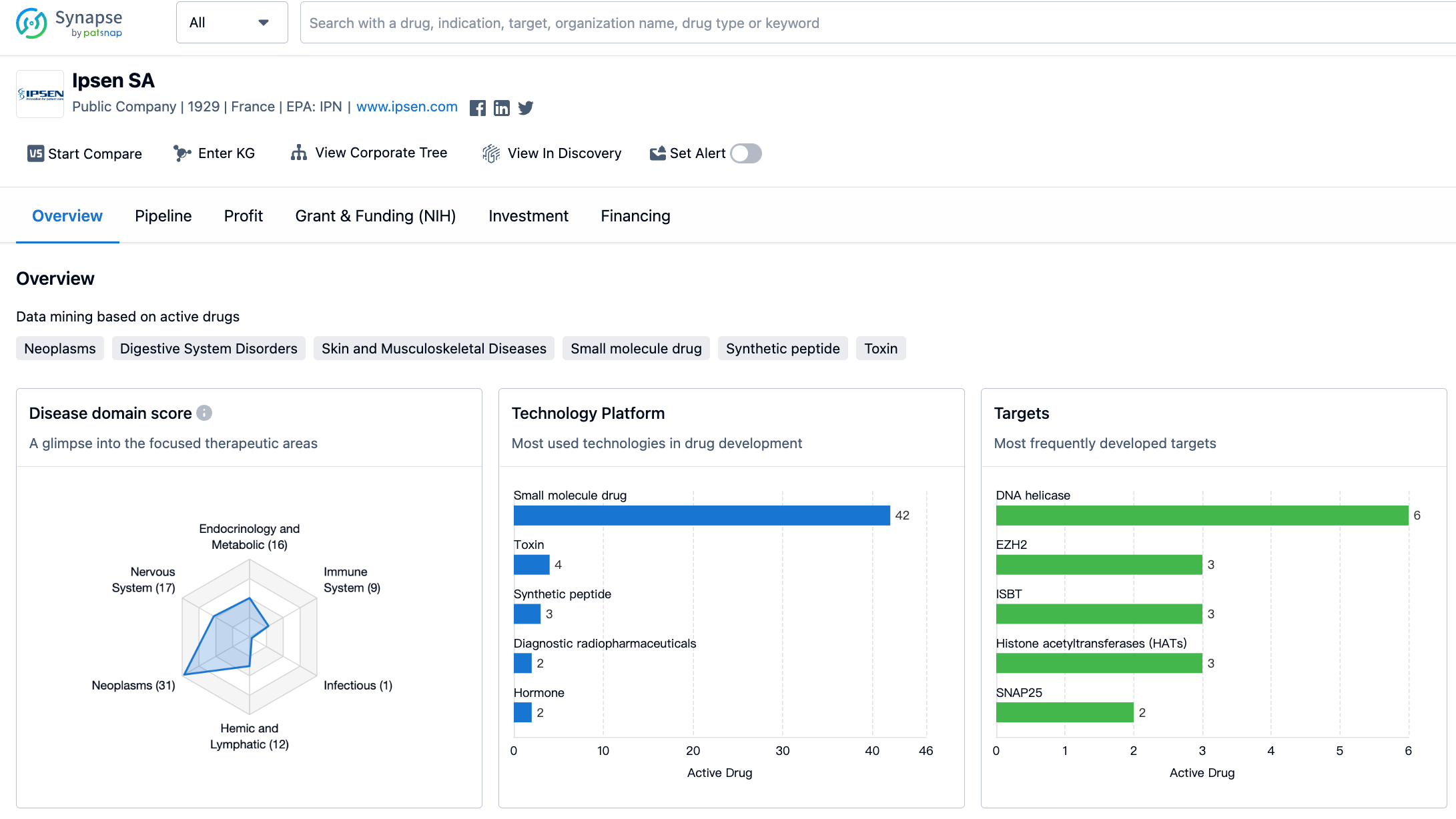
Ipsen SA has developed drugs targeting a wide range of therapeutic areas. The largest proportion of drugs is focused on the treatment of neoplasms, with a count of 31. This indicates that Ipsen SA has a strong presence in the field of oncology. The second-largest therapeutic area is nervous system diseases, with 17 drugs developed. This suggests that Ipsen SA is also actively involved in the research and development of treatments for neurological disorders. Endocrinology and metabolic diseases, as well as skin and musculoskeletal diseases, are also areas of focus for Ipsen SA, with 16 and 15 drugs developed, respectively.
Other therapeutic areas targeted by Ipsen SA include digestive system disorders, hemic and lymphatic diseases, immune system diseases, congenital disorders, cardiovascular diseases, urogenital diseases, respiratory diseases, eye diseases, otorhinolaryngologic diseases, and infectious diseases. It is worth noting that Ipsen SA has a relatively smaller number of drugs developed for these areas compared to neoplasms and nervous system diseases.
The top target is DNA helicase, with 6 drugs developed. This suggests that Ipsen SA has a strong focus on developing drugs that target this specific protein involved in DNA replication and repair processes. Other frequently developed targets include EZH2, ISBT, and histone acetyltransferases (HATs), each with 3 drugs developed. These targets are involved in various cellular processes and are often associated with diseases such as cancer and neurological disorders.
Other targets that Ipsen SA has developed drugs for include SNAP25, MC4R, DOT1L, SSTR2, NTCP, ALK2, AChR, COX-1 + COX-2, GHSR, GHR, D2 receptor + SSTR2, RARγ, GnRHR, IGF-1R, NOS2, and TOP1. These targets are involved in a wide range of biological processes and are associated with different diseases and conditions.
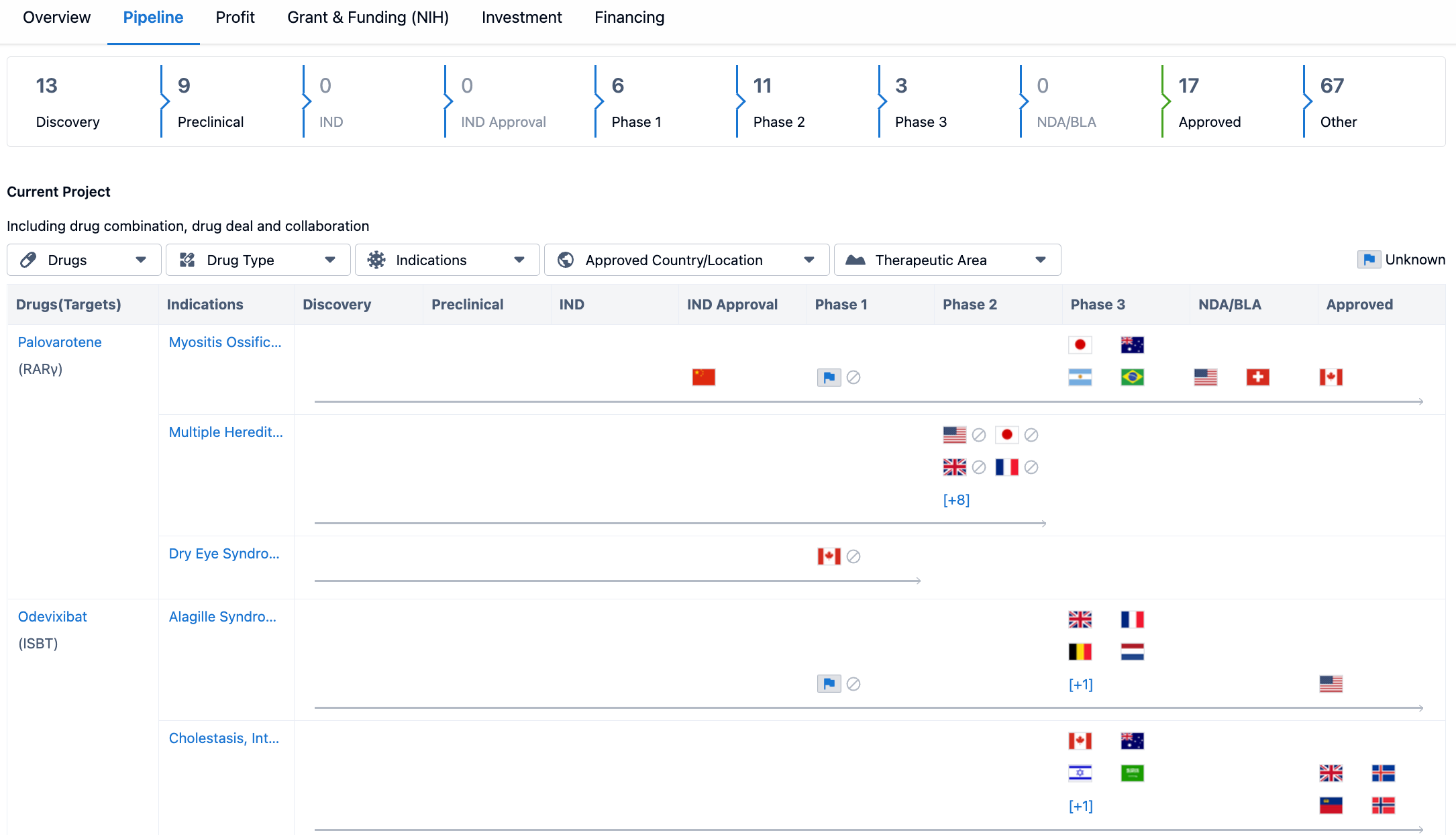
The pipeline consists of drugs in various stages of development, starting from the discovery phase to the approved phase. The discovery phase has the highest number of drugs, with 13 in total. This indicates that Ipsen SA is actively engaged in the early stages of drug development, exploring new compounds and potential therapeutic targets.
The preclinical phase follows the discovery phase, with 9 drugs in development. This phase involves testing the safety and efficacy of the drug candidates in animal models before proceeding to human trials. It is worth noting that there are no drugs in the IND (Investigational New Drug) phase or IND approval phase, indicating that Ipsen SA has not yet reached the stage of submitting investigational new drug applications to regulatory authorities.
Moving on to the clinical phases, Ipsen SA has 6 drugs in Phase 1, 11 drugs in Phase 2, and 3 drugs in Phase 3. These phases involve testing the safety and efficacy of the drug candidates in human subjects, with Phase 3 being the final stage before seeking regulatory approval. It is important to note that there are no drugs listed in the NDA/BLA (New Drug Application/Biologics License Application) phase, suggesting that Ipsen SA has not yet submitted any applications for regulatory approval.
However, Ipsen SA has a significant number of drugs listed as approved, with a count of 17. This indicates that the company has successfully obtained regulatory approval for these drugs and they are available on the market for patients. Additionally, there are 67 drugs listed under the category of "Other" in the pipeline. This category may include drugs in various stages of development or those that do not fit into the specific phases mentioned in the table.
In summary, Ipsen SA is a pharmaceutical organization with a long history in the biomedicine industry. The company has a diverse portfolio of drugs targeting various therapeutic areas, with a strong focus on neoplasms and nervous system diseases. Ipsen SA has also developed drugs targeting a wide range of molecular targets, with a particular emphasis on DNA helicase. The pipeline of Ipsen SA indicates active research and development efforts, with drugs in various stages of development, from discovery to approved. Overall, Ipsen SA demonstrates a commitment to advancing healthcare through the development of innovative pharmaceutical products.