TransCode Therapeutics Reports Promising Initial Human Trial Results for New Therapy, TTX-MC138
TransCode Therapeutics, Inc., an RNA oncology firm dedicated to revolutionizing cancer treatment with RNA-based therapies, has released early findings from its Phase 0 clinical trial involving radiolabeled TTX-MC138, indicating potential anti-tumor effects.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
New findings from a patient administered this study's treatment show that a small dose of radiolabeled TTX-MC138 led to a notable inhibition of the molecular target, miRNA-10b, in the patient’s bloodstream. Specifically, post-injection, the miR-10b levels in the patient's blood significantly dropped compared to pre-administration levels of radiolabeled TTX-MC138, achieving a 66% reduction 24 hours after dosing.
“These findings are significant because in multiple animal studies, the inhibition of miRNA-10b by TTX-MC138 resulted in complete regression of metastatic disease,” stated Zdravka Medarova, PhD, Chief Scientific Officer at TransCode. The data align with TransCode’s position that TTX-MC138’s clinical development has the potential to benefit patients with metastatic cancer.
Moreover, the research quantified the delivery amount of the drug candidate to metastatic sites, providing additional proof that TTX-MC138 accumulates in metastatic tumors. The increased radioactive lesion-to-blood ratios indicate that circulating TTX-MC138 is preferentially taken up by cancerous tissues.
“These new findings suggest TTX-MC138 not only inhibits the miRNA-10b target but is pharmacodynamically active at a single microdose in the patient’s plasma, supporting the continued clinical development of TTX-MC138 for treating various metastatic cancers in the upcoming Phase 1 clinical study. This could imply a much wider therapeutic range than previously anticipated,” said Dr. Daniel Vlock, TransCode’s Chief Medical Officer.
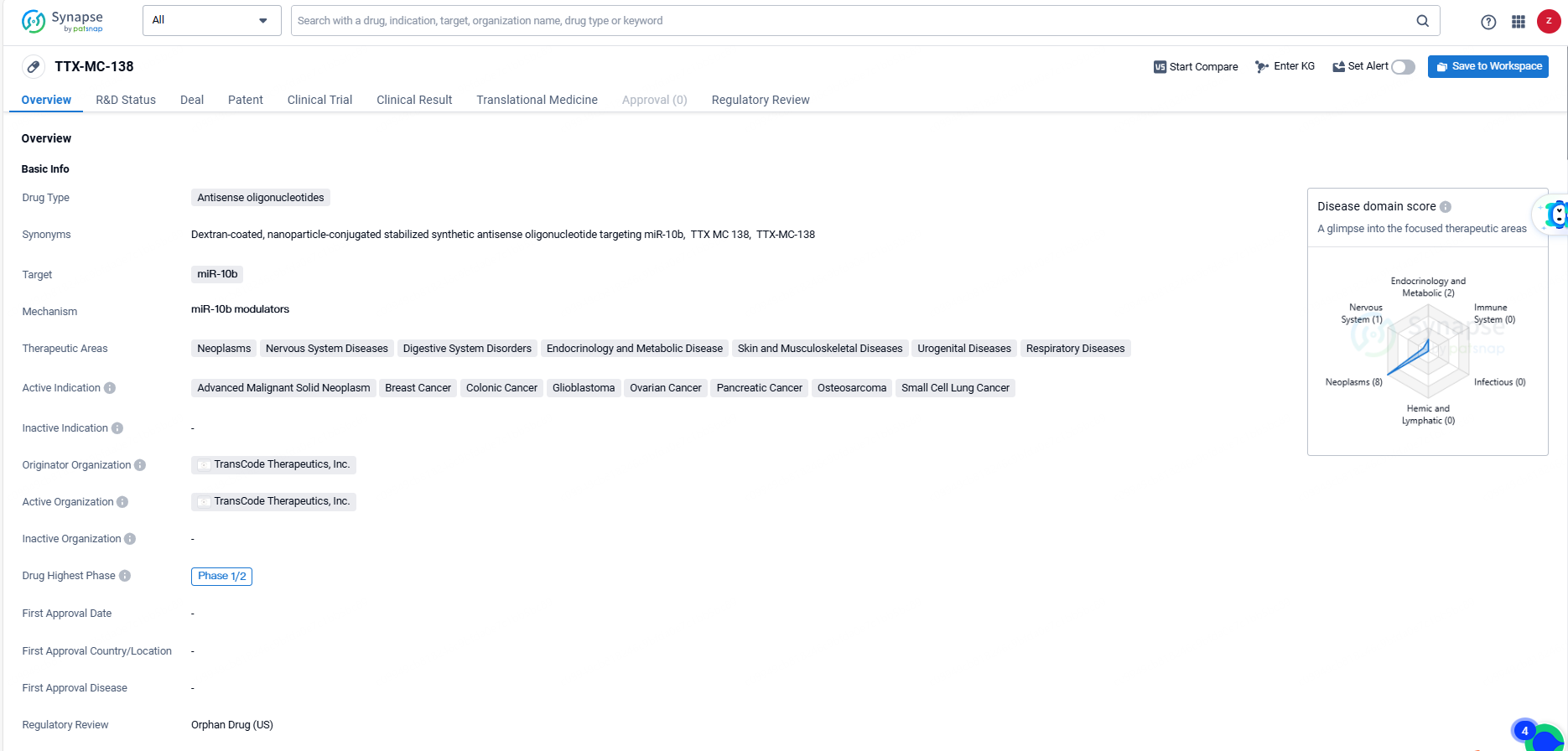
TTX-MC138, an antisense oligonucleotide drug developed by TransCode Therapeutics, Inc., targets miR-10b and is being developed to treat a broad spectrum of therapeutic areas, including neoplasms, nervous system diseases, digestive system disorders, endocrinology and metabolic diseases, skin and musculoskeletal diseases, urogenital diseases, and respiratory diseases. The active indications for TTX-MC138 include advanced malignant solid neoplasms, breast cancer, colonic cancer, glioblastoma, ovarian cancer, pancreatic cancer, osteosarcoma, and small cell lung cancer.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
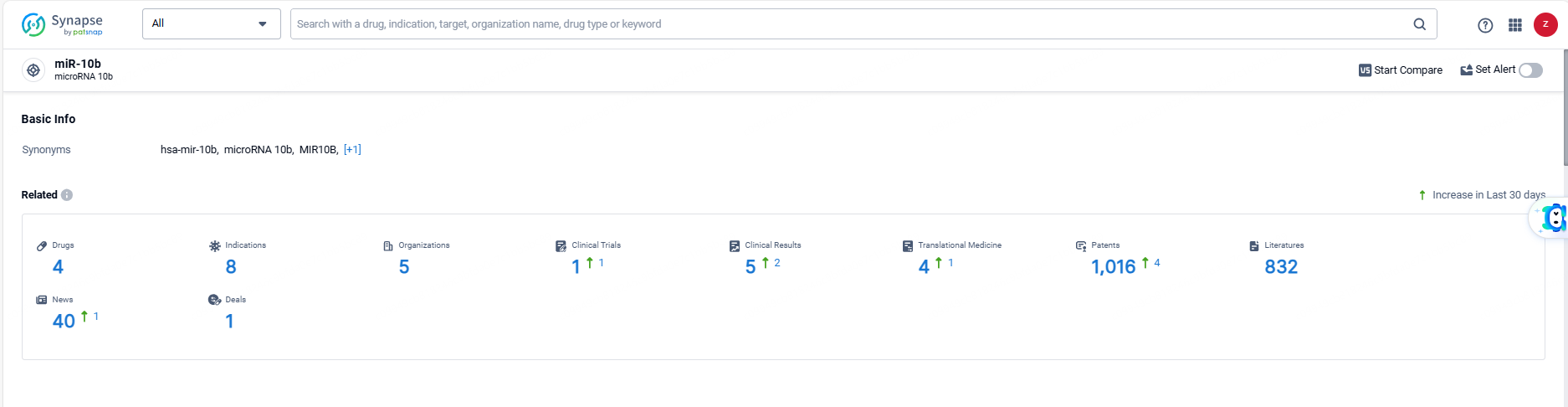
According to the data provided by the Synapse Database, As of June 6, 2024, there are 4 investigational drugs for the miR-10b targets, including 8 indications, 5 R&D institutions involved, with related clinical trials reaching 1, and as many as 1016 patents.
TTX-MC-138 has reached the highest global phase of Phase 1/2 in its development. Additionally, the drug has been designated as an orphan drug, indicating its intended use in the treatment of rare diseases or conditions.The development of TTX-MC-138 as an antisense oligonucleotide drug targeting miR-10b represents a novel approach in the field of biomedicine. The drug's broad therapeutic areas and active indications suggest its potential to address unmet medical needs across various disease categories.