Trilostane: Detailed Review of its Transformative R&D Success
Trilostane's R&D Progress
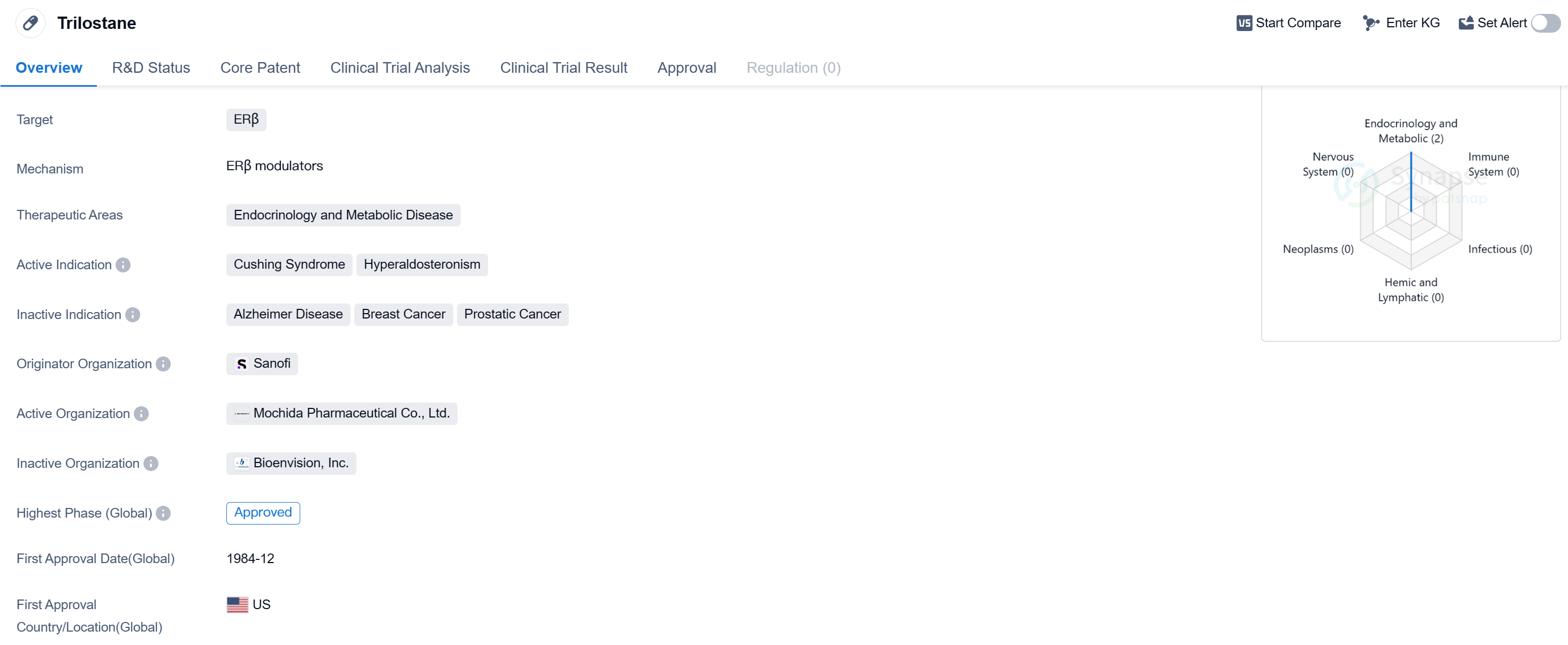
Trilostane is a small molecule drug that targets ERβ and is primarily used in the field of endocrinology and metabolic disease. It has been approved for the treatment of Cushing Syndrome and Hyperaldosteronism. The drug was first approved in the United States in December 1984.
Trilostane is developed by Sanofi, a well-known pharmaceutical organization. It is classified as a small molecule drug, indicating that it consists of low molecular weight compounds. The drug specifically targets ERβ, which stands for estrogen receptor beta. ERβ is a protein that plays a role in various physiological processes, including the regulation of gene expression.
The therapeutic areas of Trilostane are endocrinology and metabolic disease. Endocrinology focuses on the study and treatment of hormonal disorders, while metabolic disease refers to conditions that affect the body's metabolism. Trilostane is primarily used in the treatment of Cushing Syndrome and Hyperaldosteronism.
Cushing Syndrome is a hormonal disorder caused by prolonged exposure to high levels of cortisol, a stress hormone. Trilostane helps to reduce cortisol production, thereby alleviating the symptoms associated with this condition. Hyperaldosteronism, on the other hand, is a condition characterized by excessive production of aldosterone, a hormone that regulates salt and water balance. Trilostane can help to normalize aldosterone levels and manage the symptoms of Hyperaldosteronism.
Trilostane received its first approval in the United States in December 1984. The drug has also reached the highest phase of development.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Trilostane: ERβ modulators
ERβ modulators are compounds or substances that interact with and affect the activity of the estrogen receptor beta (ERβ). The estrogen receptor beta is a protein that is involved in mediating the effects of estrogen hormones in the body. It plays a role in various physiological processes, including the regulation of gene expression, cell growth, and differentiation.
ERβ modulators can have different effects on the receptor's activity. Some modulators may act as agonists, meaning they activate the receptor and mimic the effects of estrogen. Others may act as antagonists, blocking the receptor's activity and inhibiting the effects of estrogen. There are also selective modulators that can selectively activate or inhibit specific functions of the ERβ receptor.
These modulators have potential applications in biomedicine, particularly in the field of hormone-related diseases and conditions. For example, they may be used in the development of therapies for hormone-sensitive cancers, such as breast or prostate cancer, where the modulation of ERβ activity can help regulate cell growth and inhibit tumor progression. Additionally, ERβ modulators may have implications in the treatment of menopausal symptoms, osteoporosis, and cardiovascular diseases, as estrogen receptors play a crucial role in these conditions.
In summary, ERβ modulators are substances that interact with the estrogen receptor beta and can either activate or inhibit its activity, offering potential therapeutic applications in various hormone-related diseases and conditions.
Drug Target R&D Trends for Trilostane
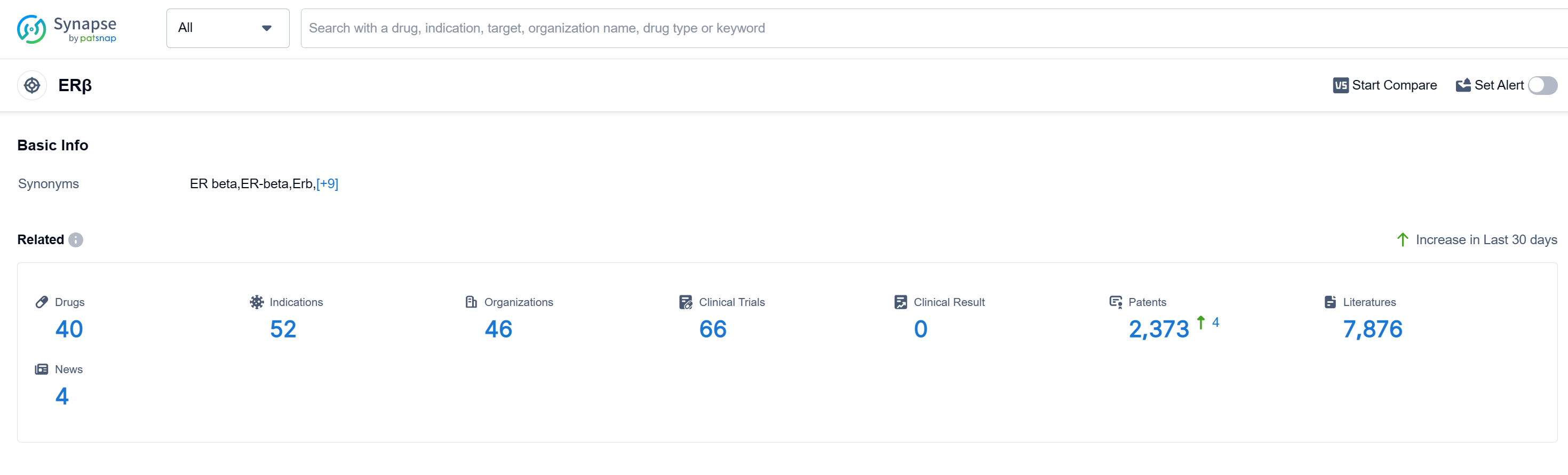
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 40 ERβ drugs worldwide, from 46 organizations, covering 52 indications, and conducting 66 clinical trials.
In conclusion, the current competitive landscape of target ERβ shows that Pfizer Inc. and Eli Lilly & Co. are leading in terms of R&D progress, with approved drugs for relevant indications. Small molecule drugs are progressing rapidly, indicating intense competition. The United States is the most active country in the development of target ERβ, followed by the European Union and Canada. China has also made progress in this field. The future development of target ERβ holds potential for further advancements in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Trilostane is a small molecule drug developed by Sanofi that targets ERβ. It is primarily used in the field of endocrinology and metabolic disease, specifically for the treatment of Cushing Syndrome and Hyperaldosteronism. The drug received its first approval in the United States in 1984 and has reached the highest phase of development.