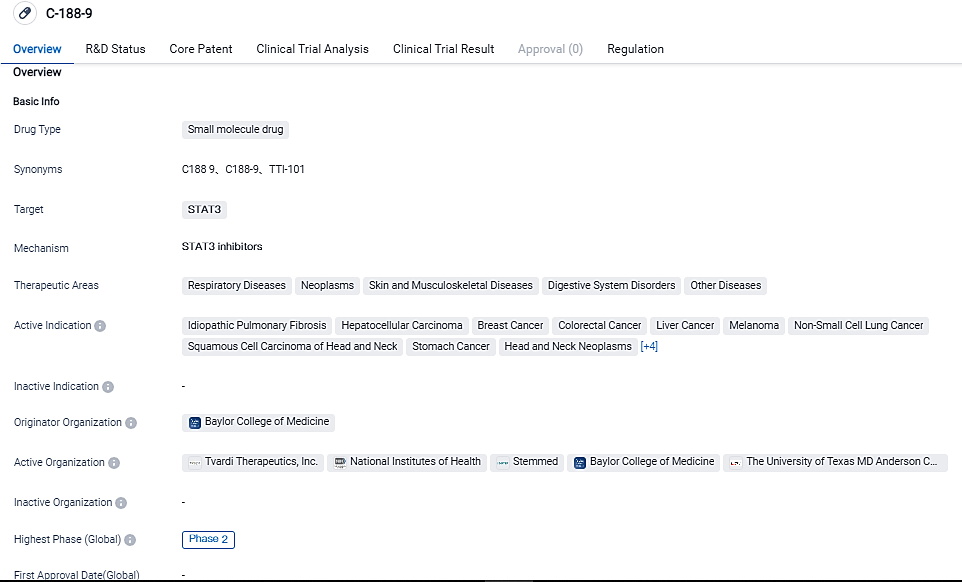
Tvardi Therapeutics Announces Phase 2 Trial of STAT3 Inhibitor TTI-101 for Idiopathic Pulmonary Fibrosis
Tvardi Therapeutics, Inc., a clinical-stage biopharmaceutical company specializing in the development of STAT3 inhibitors, has initiated dosing of the first patients in its ongoing REVERTIPF trial. This 12-week, randomized, double-blind study aims to assess the safety and clinical efficacy of TTI-101 at varying doses, either as a monotherapy or in combination with nintedanib (OFEV®), compared to placebo in patients diagnosed with idiopathic pulmonary fibrosis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive lung disease of unknown origin. It is characterized by deteriorating respiratory symptoms, declining lung function, and functional impairment. The prognosis for IPF is generally poor, as currently approved treatments only slow down the progression of the disease without being able to reverse clinical decline or restore lung function. While there are ongoing research efforts to discover new treatment approaches for IPF, none have specifically targeted STAT3, a crucial regulatory protein involved in the development of pulmonary fibrosis. TTI-101 is an orally administered small molecule that directly inhibits STAT3 and shows potential as a novel therapeutic option for IPF.
The REVERTIPF trial will provide valuable insights into the potential of STAT3 inhibition in fibrotic diseases, which have a significant impact on the lives of numerous individuals worldwide. The growing interest in our IPF study is fueled by recent clinical data demonstrating the safety and efficacy of TTI-101 in heavily treated cancer patients, as well as published preclinical research highlighting TTI-101's ability to restore fibrosis. Additionally, the FDA's Orphan Drug Designation for TTI-101 in IPF has further contributed to the enthusiasm surrounding our study. This trial marks the completion of Tvardi's three Phase 2 trials aimed at addressing diseases driven by STAT3.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
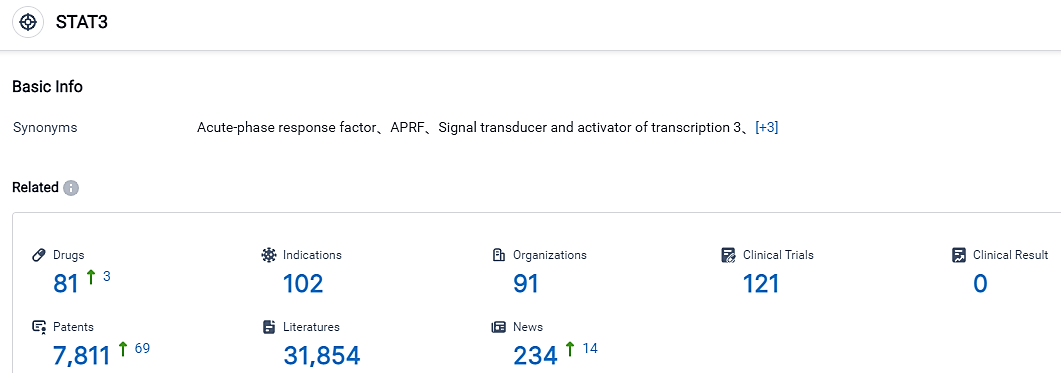 According to the data provided by the Synapse Database, As of September 2, 2023, there are 81 investigational drugs for the STAT3 target, including 102 applicable indications, 91 R&D institutions involved, with related clinical trials reaching 121, and as many as 7811 patents.
According to the data provided by the Synapse Database, As of September 2, 2023, there are 81 investigational drugs for the STAT3 target, including 102 applicable indications, 91 R&D institutions involved, with related clinical trials reaching 121, and as many as 7811 patents.
STAT3 small molecule inhibitors are drugs that target the STAT3 signaling pathway and are used for the treatment of malignant tumors, particularly gastric cancer and pancreatic cancer. STAT3 is highly expressed and persistently activated in malignant tumors, promoting the progression and metastasis of various malignant tumors by regulating genes associated with cancer cell survival, proliferation, invasion, metastasis, drug resistance, and immune evasion. Several STAT3 small molecule inhibitors have been developed, such as WB436B, and combination immunotherapy has been shown to enhance the anti-tumor efficacy and reduce adverse reactions. The research on targeted STAT3 small molecule inhibitors for anti-tumor therapy has made significant progress and is a hot topic in anti-cancer drug development.