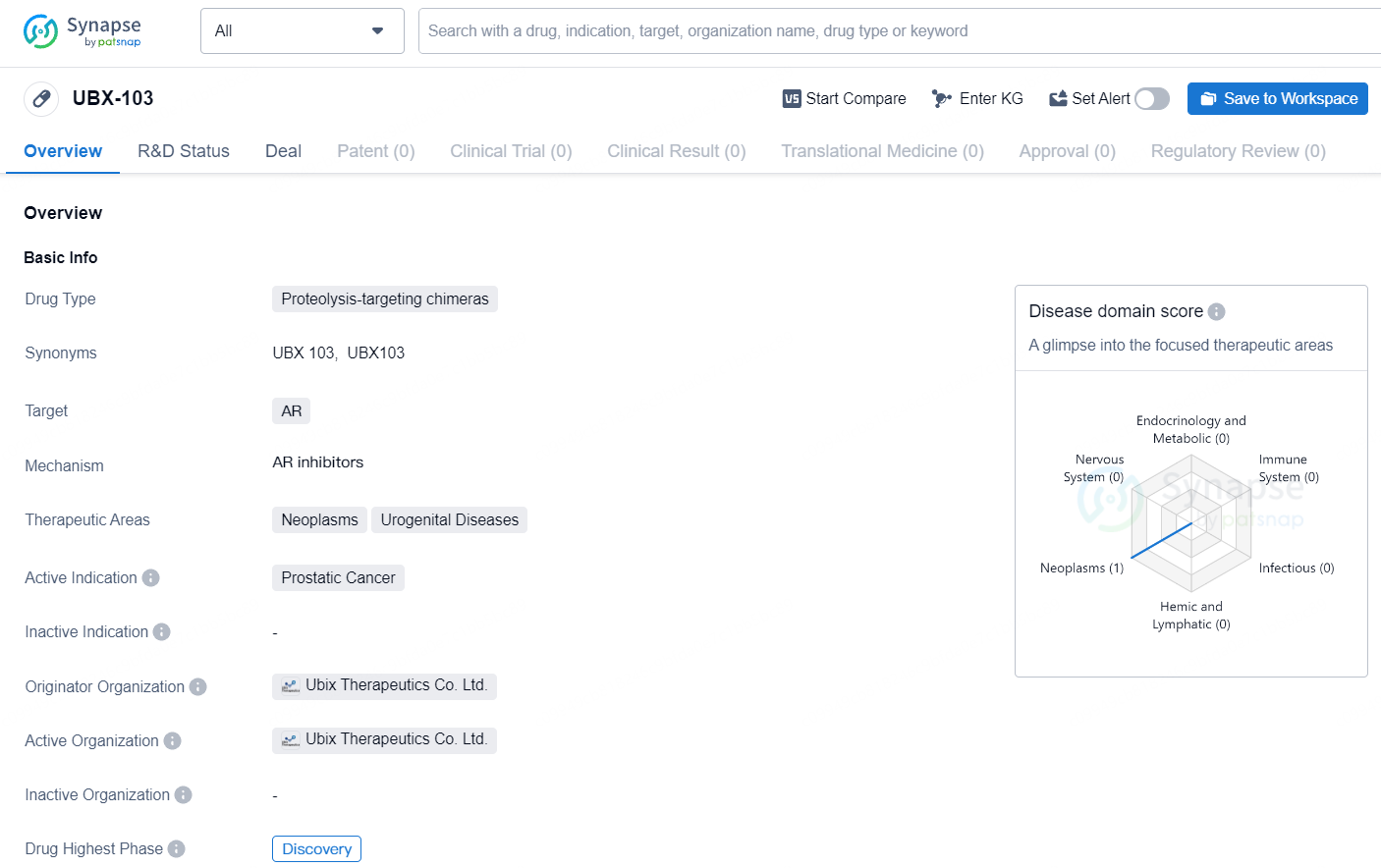
Ubix Therapeutics and Yuhan Announce Exclusive Licensing for Oral AR Degrader UBX-103 to Combat Advanced Prostate Cancer
Ubix Therapeutics, Inc., a biotech firm focused on discovering and creating advanced oncology treatments utilizing targeted protein degradation, has announced an exclusive licensing deal with Yuhan. This agreement aims to develop and market the preclinical androgen receptor (AR) degrader program, UBX-103.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
As per the agreement, Yuhan has obtained exclusive global rights for the preclinical and clinical development, as well as the commercialization, of UBX-103. Ubix will receive an initial payment exceeding 3.6 million US dollars. Additionally, Ubix could receive up to 105 million US dollars through various developmental, regulatory, and commercial milestones and will also benefit from high single-digit royalty payments. Should a sublicensing deal with a third party occur, Ubix will be entitled to a share of the profits.
UBX-303-1 is a promising orally bioavailable AR degrader that targets the androgen receptor, crucial for the proliferation and survival of prostate cancer cells. Although hormone deprivation therapy is typically used for treating metastatic prostate cancer, tumors often evolve into castration-resistant metastatic prostate cancer due to AR gene amplification or treatment-induced gain-of-function mutations.
Preclinical studies have shown that UBX-103 is a potent AR degrader, with a picomolar DC50, and is effective against a wide range of treatment-induced resistance mutations. Remarkably, UBX-103 has demonstrated the capacity to impede the growth of tumors that are resistant to next-generation hormonal therapies in in vivo preclinical models.
"Yuhan and Ubix will enhance their collaboration and technology exchange to create next-generation therapies utilizing targeted protein degradation," stated Wook-Je Cho, CEO of Yuhan. "This collaboration aspires to provide better treatment options for patients who have limited choices with current therapies while driving innovation in medical and life sciences."
BK Seo, CEO of Ubix Therapeutics, commented, "Yuhan is a prominent pharmaceutical company recognized globally for its R&D excellence and the commercialization of innovative medicines. Through this collaboration, we believe that Yuhan's expertise and dedication will significantly advance the development and potential commercialization of UBX-103."
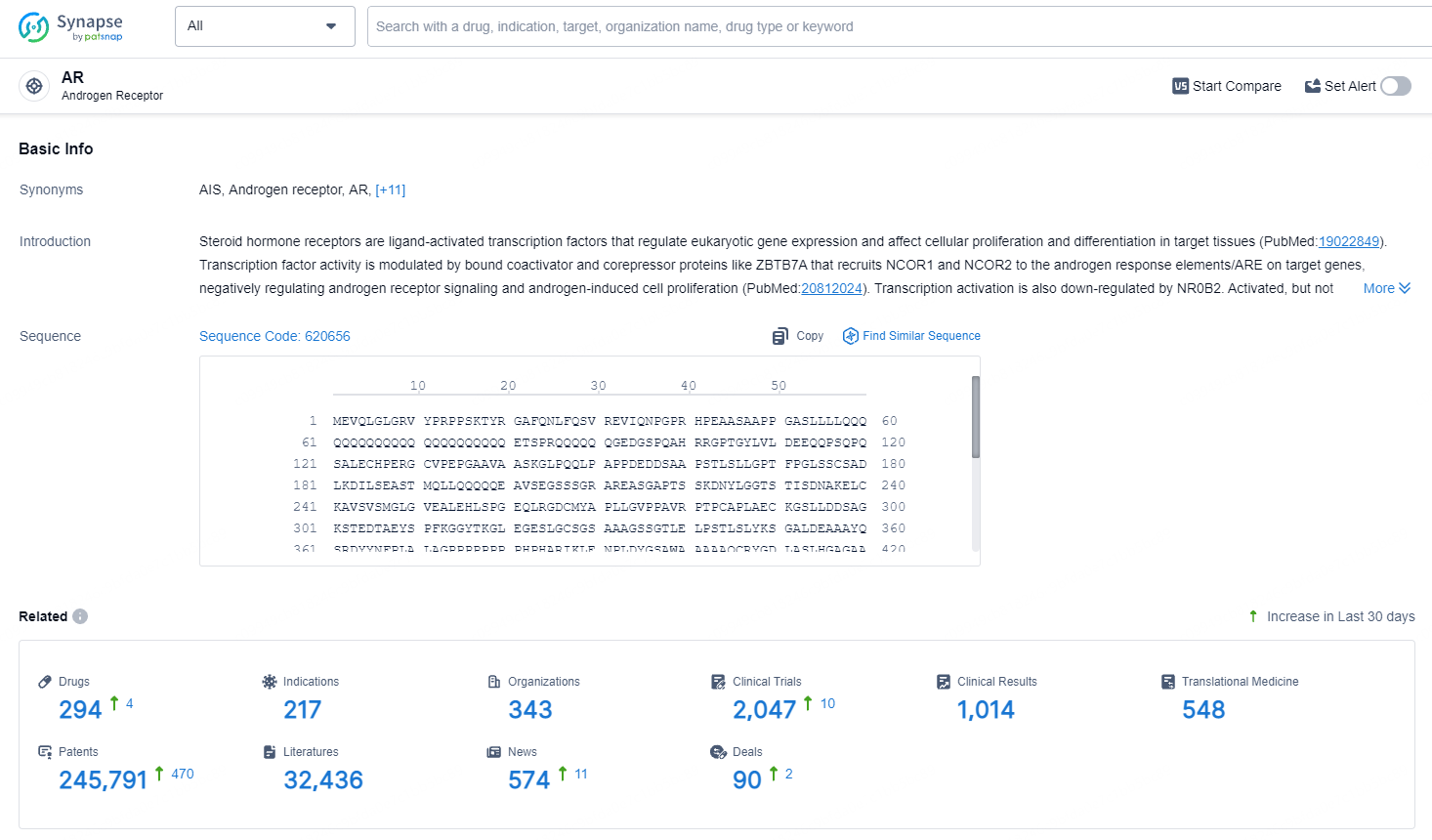
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of July 5, 2024, there are 294 investigational drugs for the AR target, including 217 indications, 343 R&D institutions involved, with related clinical trials reaching 2047, and as many as 245791 patents.
UBX-103 represents a promising new approach in the field of biomedicine for the treatment of prostatic cancer. However, it is important to note that the drug is still in the early stages of development, and further research and development efforts will be needed to determine its potential as a future treatment option for patients.