EU Approves First Sanofi-Regeneron Drug, Dulprizumab for COPD
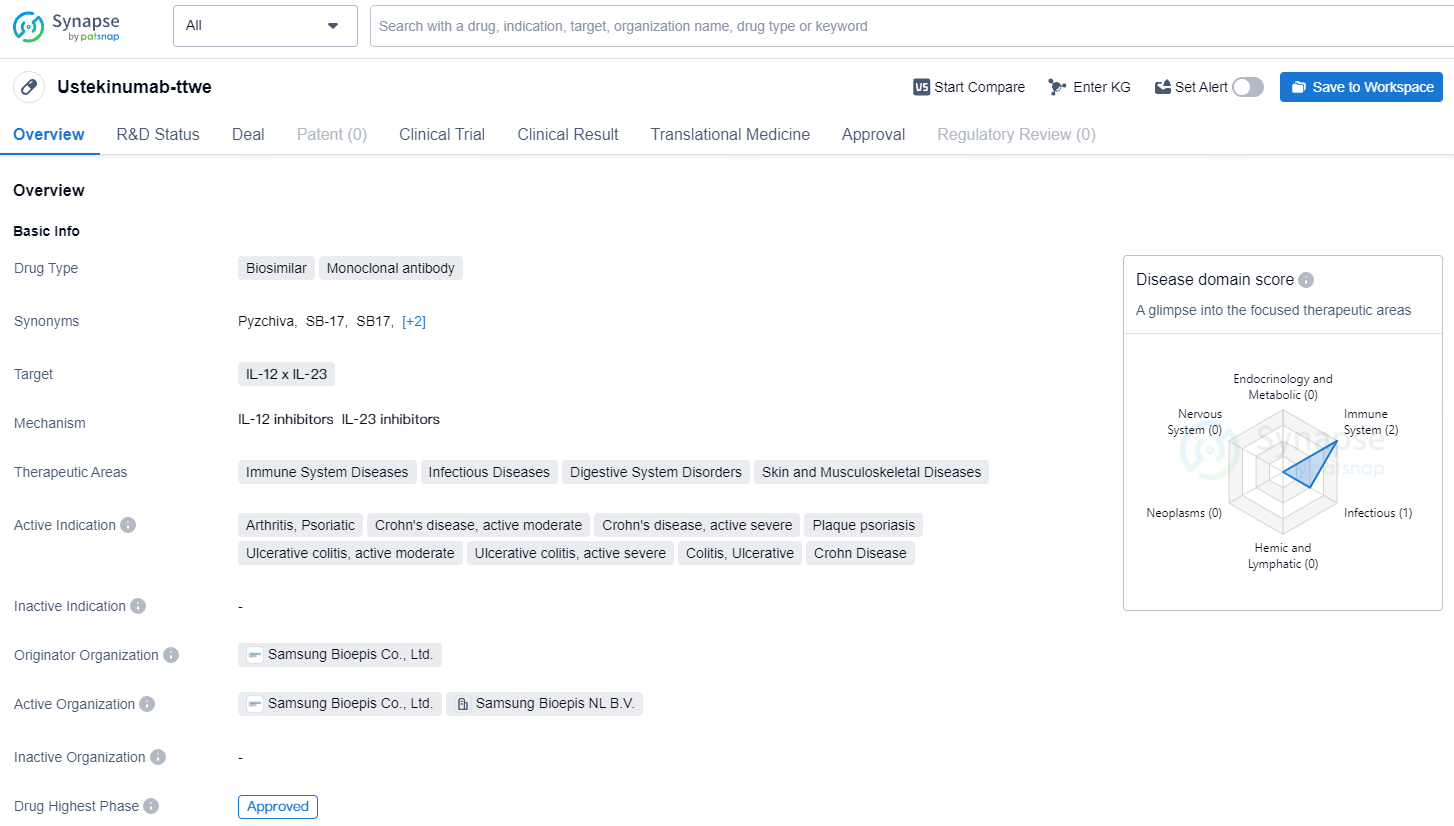
Samsung Bioepis Co., Ltd. revealed that the U.S. Food and Drug Administration (FDA) has granted approval for the Biologics License Application for PYZCHIVA® (ustekinumab-ttwe), available in both subcutaneous injection and intravenous infusion forms, as a biosimilar to Stelara.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
PYZCHIVA has received approval for the management of moderate to severe plaque psoriasis in patients eligible for phototherapy or systemic therapy, active psoriatic arthritis, moderately to severely active Crohn’s disease, and moderately to severely active ulcerative colitis. Additionally, PYZCHIVA has been granted provisional determination for interchangeability.
"The FDA’s approval of PYZCHIVA as a biosimilar to Stelara marks a significant step forward for individuals dealing with inflammatory conditions, as biosimilars can provide more treatment options and enhance access to biologic therapies," stated Byoung In Jung, Vice President and Head of Regulatory Affairs at Samsung Bioepis.
"Biosimilars also have the potential to alleviate the economic pressures on healthcare systems, particularly in the US where biologics represent over 46% of the annual pharmaceutical expenditure. We remain committed to broadening access to medications by advancing our biosimilar portfolio to benefit patients, healthcare providers, and healthcare infrastructures globally," she added.
The FDA’s authorization of PYZCHIVA is supported by comprehensive evidence, including analytical, non-clinical, and clinical data that confirm its biosimilarity to Stelara, without any significant clinical differences in safety, purity, and potency. A Phase 1 clinical study, which was randomized, double-blind, and included three arms in a parallel-group, single-dose setup, showcased the pharmacokinetic equivalence and similar safety, tolerability, and immunogenicity profiles between PYZCHIVA and Stelara in healthy participants.
A Phase 3 clinical trial, which was randomized, double-blind, and multicenter, was conducted in patients with moderate to severe plaque psoriasis. It demonstrated the biosimilarity of SB17 to Stelara, showing equivalent efficacy and comparable safety and pharmacokinetic profiles up to Week 28. These primary findings were published in the Journal of the American Academy of Dermatology.
Developed by Samsung Bioepis, PYZCHIVA will be marketed in the United States by Sandoz. Samsung Bioepis and Sandoz signed a commercialization agreement for SB17 in September 2023, covering the US, Canada, European Economic Area, Switzerland, and the United Kingdom. In the US, the licensing period for PYZCHIVA will commence on February 22, 2025, in accordance with the settlement and license agreement between Samsung Bioepis and Janssen Biotech Inc.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
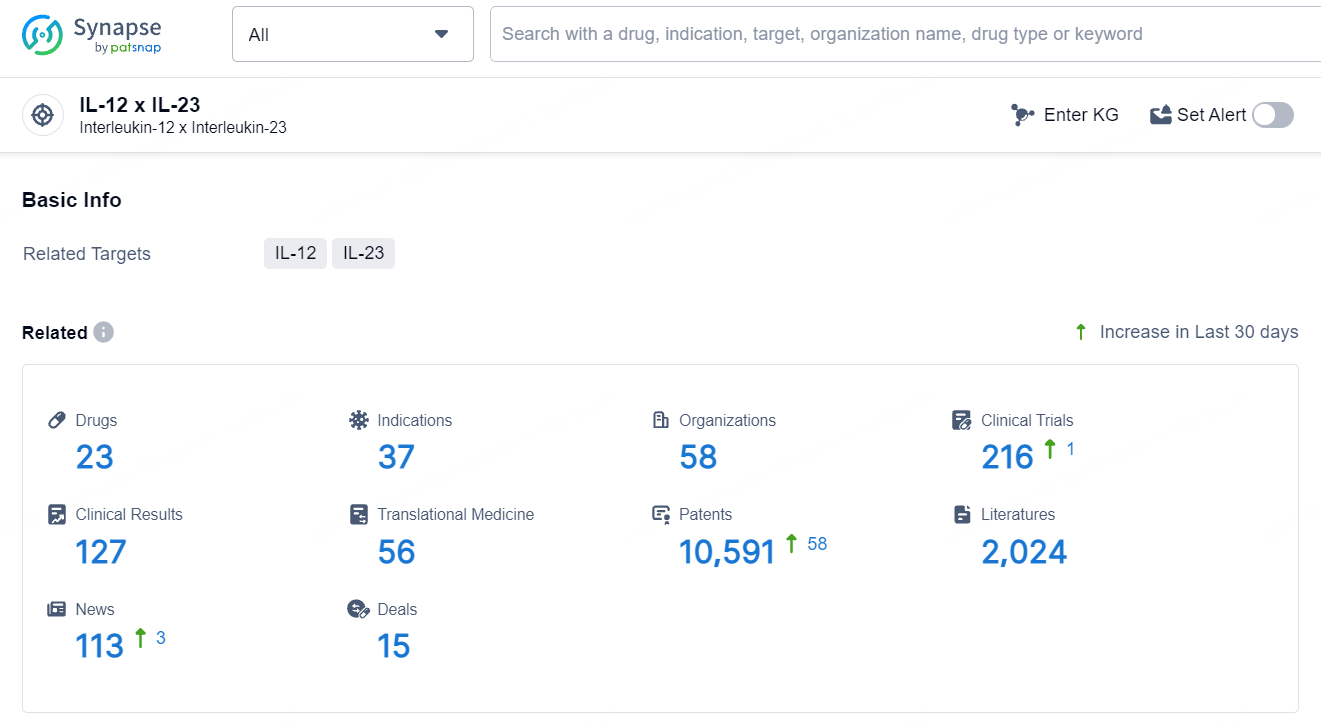
According to the data provided by the Synapse Database, As of July 5, 2024, there are 23 investigational drugs for the IL-12 and IL-23 target, including 37 indications, 58 R&D institutions involved, with related clinical trials reaching 216, and as many as 10591 patents.
Ustekinumab-ttwe is a biosimilar drug classified as a monoclonal antibody that targets IL-12 x IL-23. It is indicated for the treatment of various immune system diseases, infectious diseases, digestive system disorders, and skin and musculoskeletal diseases. The approval of Ustekinumab-ttwe represents a positive development in the field of biomedicine, providing healthcare professionals and patients with a new option for managing immune system diseases, infectious diseases, and digestive system disorders.