UK's initial participant receives OATD-01 treatment in Phase 2 of the KITE trial targeting lung sarcoidosis
Molecure S.A., an innovative biotech enterprise specializing in the invention and advancement of pharmaceutical compounds up to the clinical phase, leverages its unparalleled skill set in the domains of medicinal chemistry and biology. The firm is dedicated to pioneering the creation of novel small-molecule medications that precisely regulate protein functions and the process of mRNA translation. These treatments aim at combating various diseases that are currently deemed untreatable. The company has initiated a Phase II clinical study, where the investigative drug OATD-01 is being provided to individuals diagnosed with active pulmonary sarcoidosis for assessment.
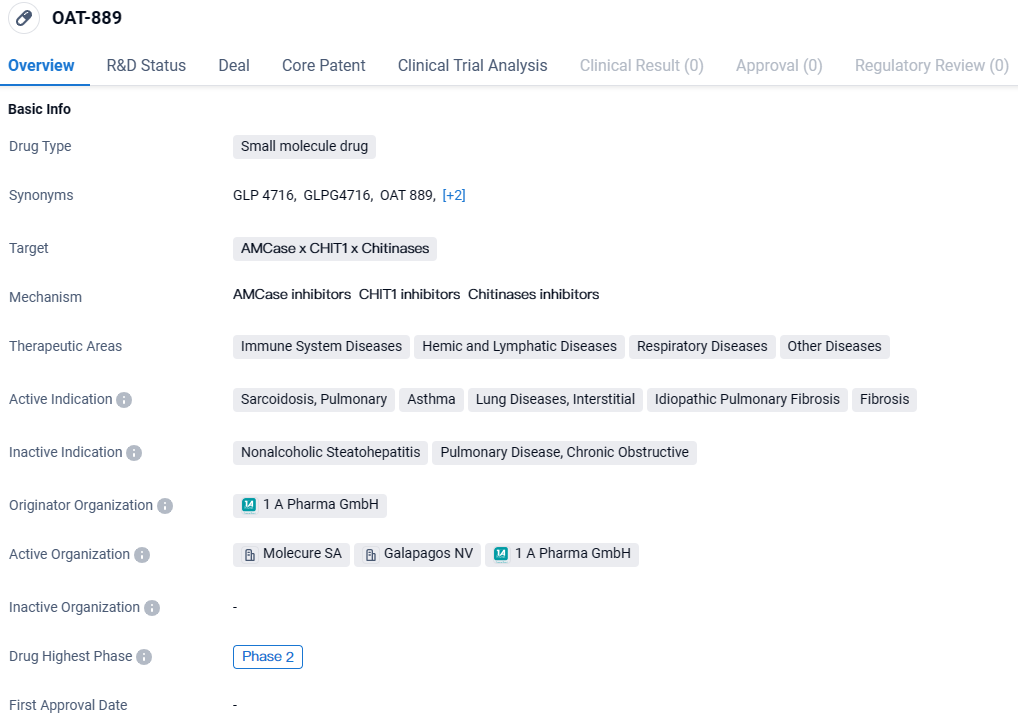
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The inaugural treatment using the chitotriosidase 1 (CHIT1) inhibitor was administered to a patient at the Edinburgh Royal Infirmary. In this research, participants are assigned a consistent dosage of 25 mg of the OATD-01 medication or a matched placebo, to be taken once daily for a duration of 12 weeks. To ensure participant welfare, thorough monitoring practices will be employed, incorporating regular medical laboratory assessments, neurologic evaluations, and heart and lung tests using ECG and spirometry.
The second phase of the OATD-01 clinical study is structured as a randomized, double-blind, placebo-controlled trial spread across multiple sites and aims to assess the therapeutic impact and safety profile of the oral CHIT1 inhibitor in a group of roughly 100 patients with diagnosed active pulmonary sarcoidosis. This patient group includes individuals who have and have not been treated with other medicinal therapies previously.
About 20 to 30 research locations are anticipated to join this international study, spanning across the United States, the European Union, Norway, and the United Kingdom. The esteemed Contract Research Organization (CRO) Simbec Orion has been tasked with overseeing and managing the details of the research process.
With the obligation to adhere to stringent double-blinding protocol, the release of the comprehensive unmasked outcomes will be aligned with the conclusion of the research and is projected by the end of the year 2025. A novel endpoint, acknowledged by the FDA for the effectiveness trial, is the analysis of the response to the 12-week treatment regimen with OATD-01, focusing on the scale of the decline in granulomatous tissue inflammation within the lungs, which will be quantified by PET/CT scans.
When the study advances to the point where around 50 of its patients have completed their involvement, an interim analysis will take place. An impartial committee will be appointed to statistically scrutinize the findings and make an informed determination on how to proceed with the rest of the study, considering patient volume adjustments.
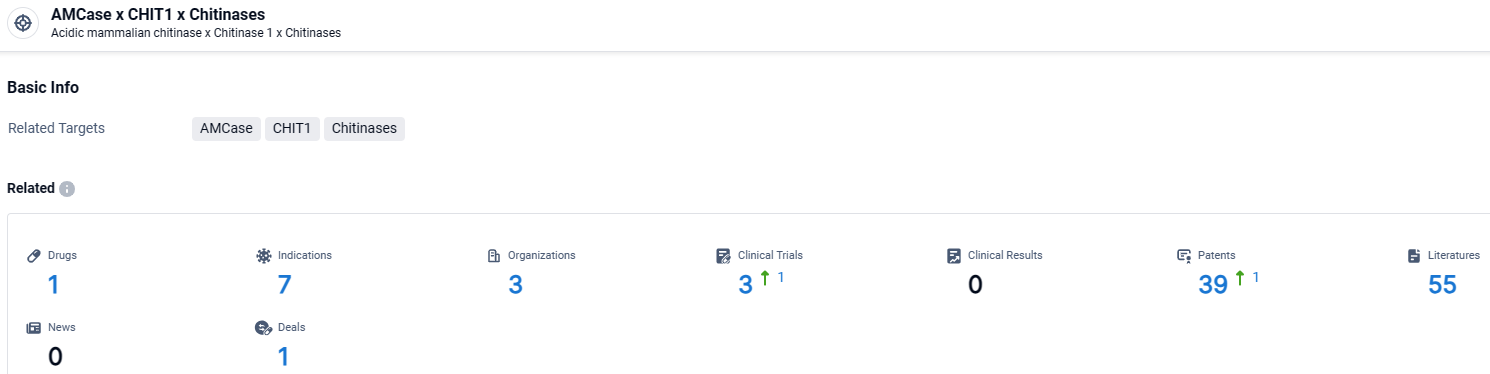
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 25 2024, there are 1 investigational drugs for the Chitinases x CHIT1 and AMCase target, including 7 indications, 3 R&D institutions involved, with related clinical trials reaching 3, and as many as 39 patents.
OATD-01 shows potential as a small molecule drug for the treatment of various diseases, particularly those related to the immune system, respiratory system, and fibrosis. Further research and development are needed to fully understand its mechanism of action and assess its safety and efficacy in larger patient populations.