Ultomiris Approved by US to Treat Adults with NMOSD
Ultomiris (ravulizumab-cwvz), has received authorization from health authorities in the U.S. to be the inaugural and unique extended-duration C5 complement inhibitor indicated for therapy in grown-up individuals diagnosed with neuromyelitis optica spectrum disorder that is linked to the presence of anti-aquaporin-4 antibodies.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The US Food and Drug Administration granted authorization for the drug following the favorable data emanating from Stage III of the CHAMPION-NMOSD trial. This data was detailed in the medical journal Annals of Neurology. Within this clinical study, Ultomiris's efficacy was assessed against a control group using a placebo from the key Soliris PREVENT study.
Neuromyelitis Optica Spectrum Disorder (NMOSD) is an uncommon, severely incapacitating disorder of the autoimmune system, influencing the brain's, spine's, and optic nerves' functioning. Individuals affected by NMOSD commonly struggle with unpredictable flare-ups, marked by either the emergence of new neurological indicators or the exacerbation of pre-existing ones. These episodes, which can be acute and often reoccur, have the potential to cause lasting impairments. In the United States, it is projected that around 6,000 adult individuals are diagnosed with NMOSD.
Mayo Clinic expert Sean J. Pittock, MD, serving as the director for both the Multiple Sclerosis and Autoimmune Neurology Center and the Neuroimmunology Laboratory, remarked, “Blocking the activity of the C5 protein within the immune system's complement pathway has been established as an effective strategy to diminish the likelihood of NMOSD attacks. This works by preventing assaults on the healthy nerve cells located in the brain, spinal cord, and optic nerve.”
Marc Dunoyer, the CEO of Alexion Pharmaceuticals, commented, "Our company has consistently led in the NMOSD field, working tirelessly to shield patients from the dread of devastating, possibly fatal flare-ups. Leveraging the proven benefits of C5 inhibition for patients diagnosed with AQP4 Ab+ NMOSD, we are elated to provide an innovative, extended-duration therapy that could potentially avert flare-ups, administered via a manageable bi-monthly dosage regimen."
As per the findings from the CHAMPION-NMOSD clinical trial, the safety profile of Ultomiris was in agreement with outcomes from past study trials and its application in clinical settings, with no unforeseen safety concerns. The frequently encountered side effects involved COVID-19 infections, cephalalgia, dorsalgia, joint discomfort, and infections of the urinary system.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
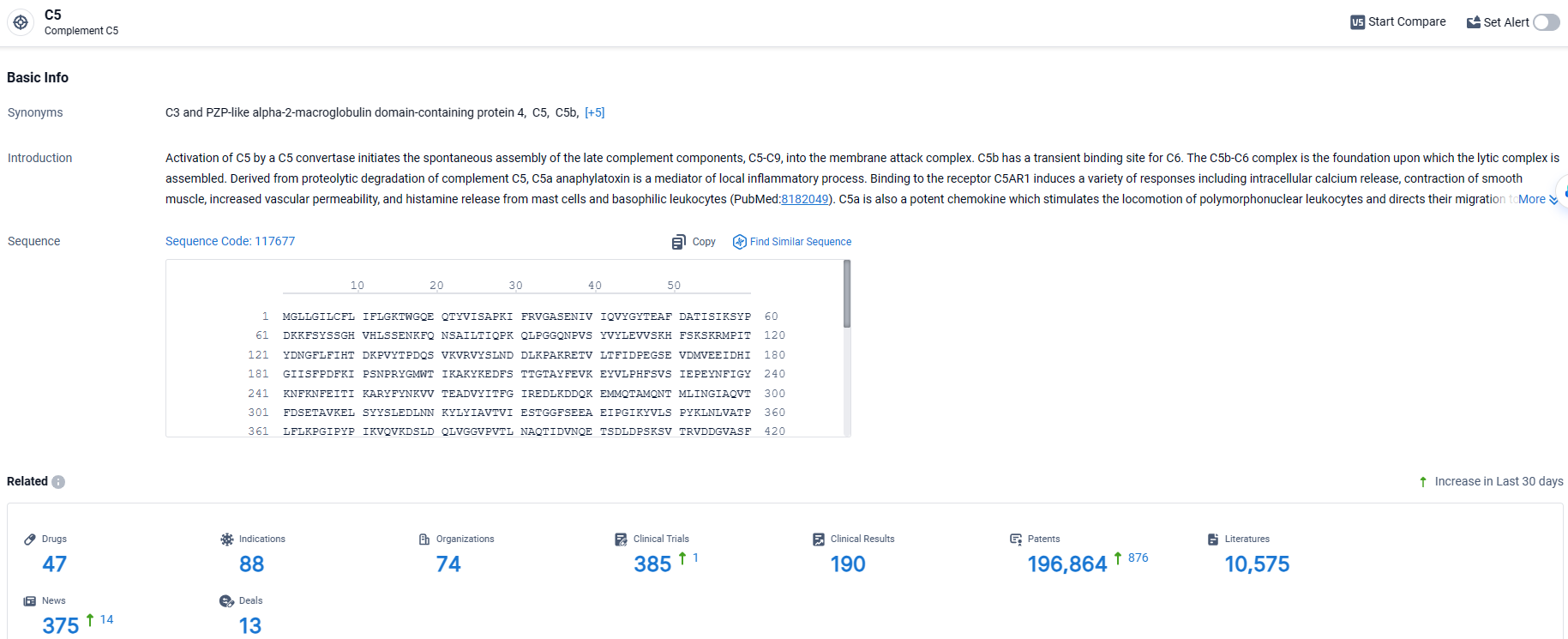
According to the data provided by the Synapse Database, As of March 26 2024, there are 47 investigational drugs for the C5 target, including 88 indications, 74 R&D institutions involved, with related clinical trials reaching 385, and as many as 196864 patents.
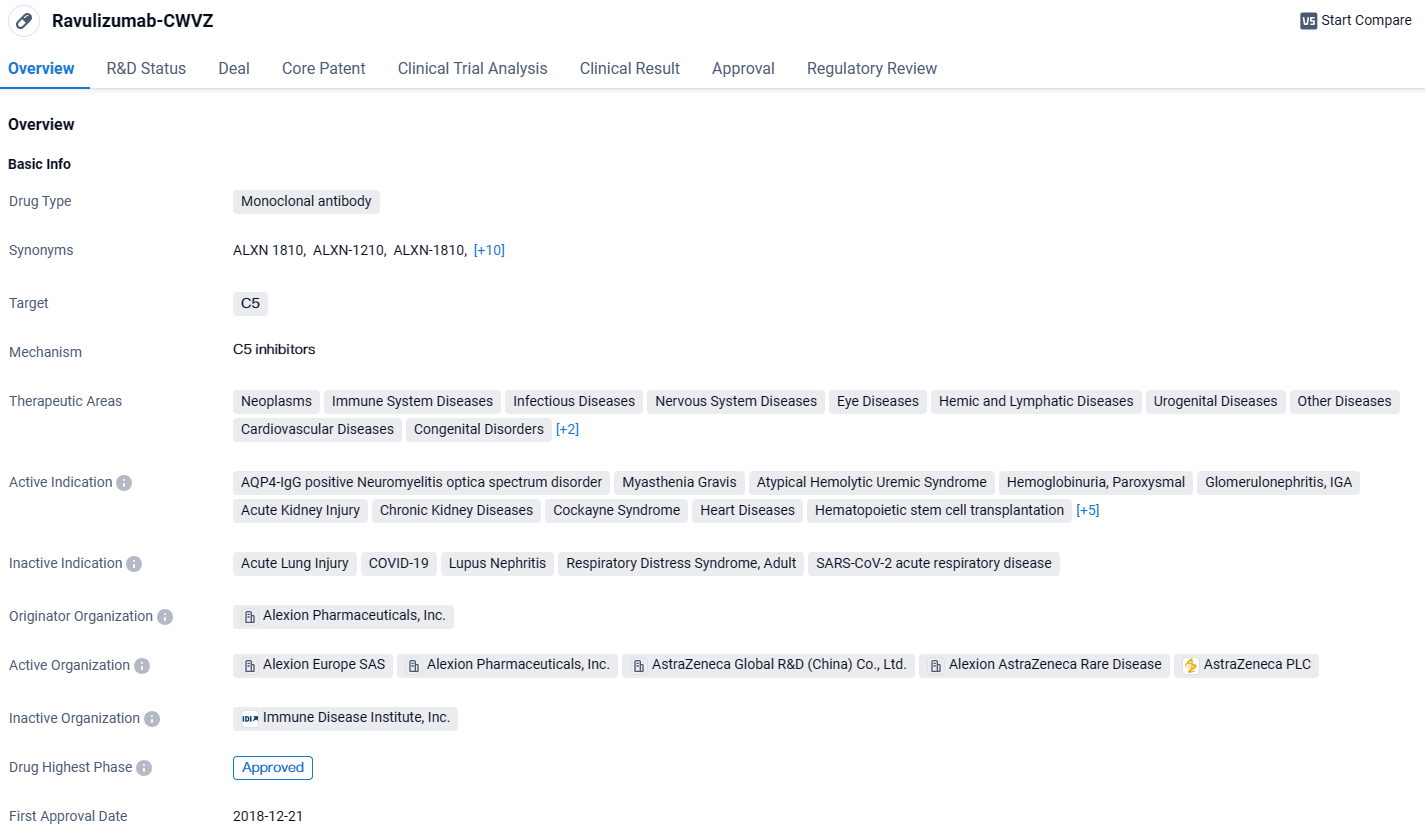
Ravulizumab-CWVZ has received approval for various therapeutic areas and has shown efficacy in treating a wide range of diseases. With its highest phase of approval globally and ongoing clinical trials in China, Ravulizumab-CWVZ holds promise for patients in need of effective treatment options.