Unleashing the Power of Flumazenil: A Comprehensive Review on R&D Breakthroughs
Flumazenil's R&D Progress
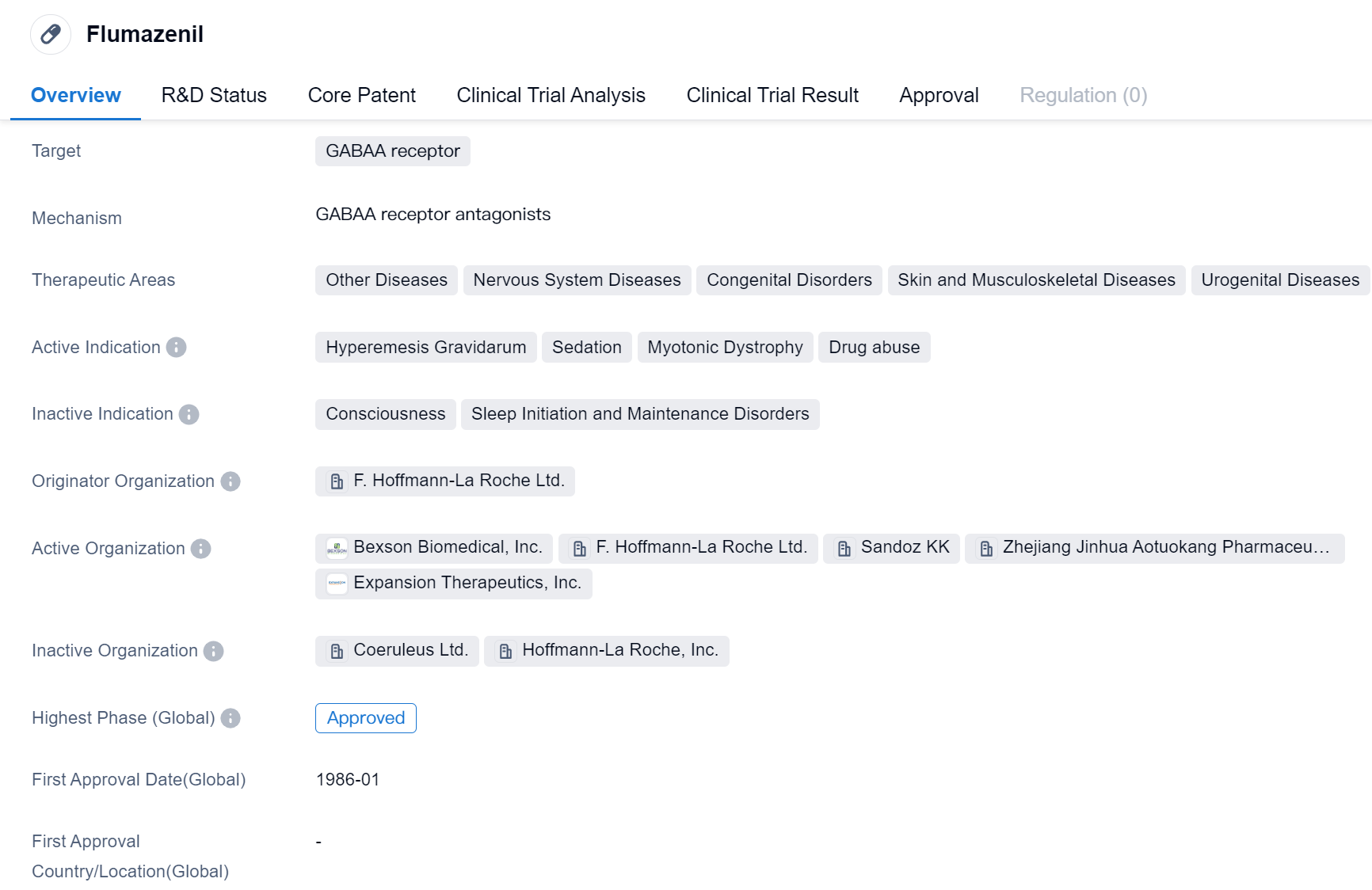
Flumazenil is a small molecule drug that targets the GABAA receptor. It has been approved for use in various therapeutic areas, including Other Diseases, Nervous System Diseases, Congenital Disorders, Skin and Musculoskeletal Diseases, and Urogenital Diseases. The drug is indicated for the treatment of Hyperemesis Gravidarum, Sedation, Myotonic Dystrophy, and Drug abuse.
Flumazenil was developed by F. Hoffmann-La Roche Ltd., a pharmaceutical company known for its contributions to the field of biomedicine. The drug has reached the highest phase of development which is approved globally, indicating its widespread recognition and acceptance.
The first approval of Flumazenil in the global market took place in January 1986. Since then, it has been used to address various medical conditions related to the nervous system, congenital disorders, and other diseases. Hyperemesis Gravidarum, a condition characterized by severe nausea and vomiting during pregnancy, is one of the approved indications for Flumazenil. The drug has also been found effective in managing sedation, myotonic dystrophy, and drug abuse.
As a small molecule drug, Flumazenil acts on the GABAA receptor, which plays a crucial role in the central nervous system. By targeting this receptor, the drug modulates the activity of gamma-aminobutyric acid (GABA), a neurotransmitter that inhibits neuronal activity. This mechanism of action allows Flumazenil to counteract the effects of excessive sedation or the misuse of certain drugs.
The highest R&D phase of Flumazenil is approved. Its long history of use since 1986 further supports its reliability as a therapeutic option. The drug's broad therapeutic areas indicate its potential to address a range of diseases and conditions beyond its primary indications.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Flumazenil: GABAA receptor antagonists
From a biomedical perspective, GABAA receptor antagonists are a class of drugs that specifically target and block the activity of GABAA receptors in the central nervous system. GABAA receptors are a type of neurotransmitter receptor that bind to the neurotransmitter gamma-aminobutyric acid (GABA), which is an inhibitory neurotransmitter. By antagonizing or inhibiting the GABAA receptors, these drugs can modulate the excitability of neurons and affect various physiological processes in the brain.
GABAA receptor antagonists have diverse medical applications. They are commonly used as sedatives, anxiolytics (anti-anxiety medications), and hypnotics (sleep-inducing medications). These drugs can help reduce anxiety, induce relaxation, and promote sleep by inhibiting the inhibitory actions of GABA, leading to an overall increase in neuronal activity.
Additionally, GABAA receptor antagonists are sometimes used as anticonvulsants to prevent or reduce seizures. By blocking the inhibitory effects of GABA, these drugs can help regulate abnormal electrical activity in the brain and prevent the spread of seizures.
It's worth noting that GABAA receptor antagonists can have various side effects, including drowsiness, dizziness, impaired coordination, and cognitive impairment. Therefore, they should be used under medical supervision and prescribed at appropriate doses to minimize potential risks.
Drug Target R&D Trends for Flumazenil
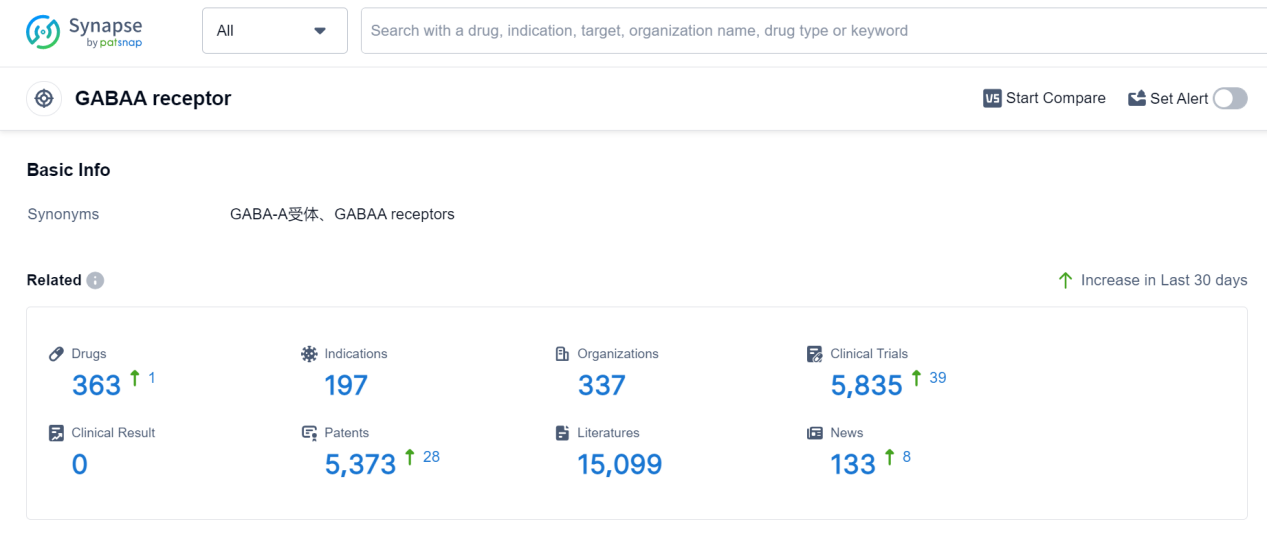
The GABAA receptor is a crucial component of the human body's central nervous system. It is a type of neurotransmitter receptor that binds to the neurotransmitter gamma-aminobutyric acid (GABA). Activation of the GABAA receptor leads to the inhibition of neuronal activity, resulting in a calming and sedative effect. This receptor plays a vital role in regulating anxiety, sleep, muscle relaxation, and the prevention of seizures. Additionally, it is the target of various pharmaceutical drugs, such as benzodiazepines, which enhance the receptor's activity to treat conditions like anxiety disorders, insomnia, and epilepsy. Understanding the role of the GABAA receptor is essential for developing effective medications that modulate its function.
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 363 GABAA receptor drugs worldwide, from 337 organizations, covering 197 indications, and conducting 5835 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Flumazenil is a small molecule drug developed by F. Hoffmann-La Roche Ltd. It targets the GABAA receptor and has been approved for use in various therapeutic areas, including Other Diseases, Nervous System Diseases, Congenital Disorders, Skin and Musculoskeletal Diseases, and Urogenital Diseases. The drug is indicated for the treatment of Hyperemesis Gravidarum, Sedation, Myotonic Dystrophy, and Drug abuse. Its approval in the global market, along with its long history of use, underscores its significance in the field of biomedicine.