Unleashing the Power of tamibarotene: A Comprehensive Review on R&D Breakthroughs
Tamibarotene's R&D Progress
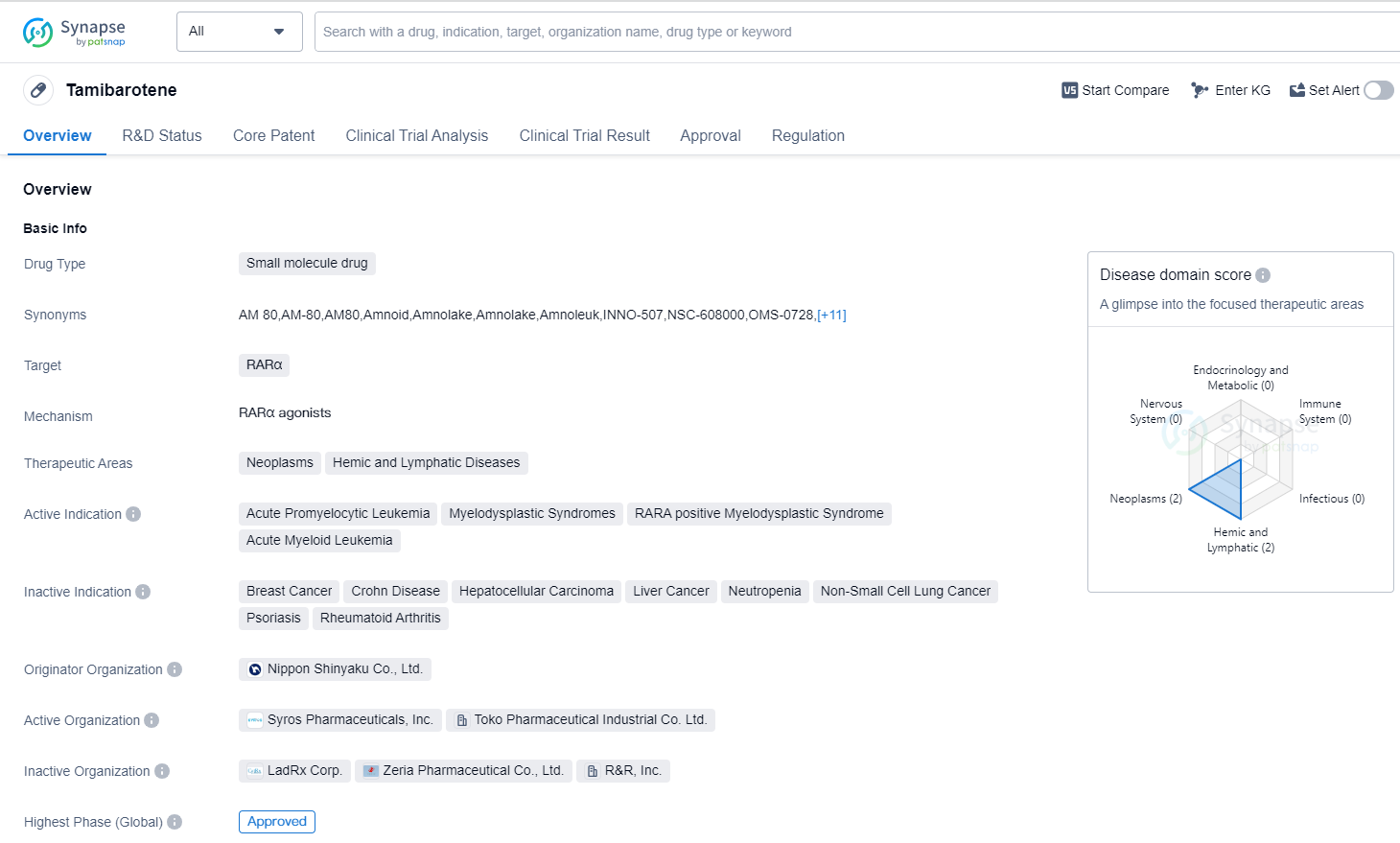
Tamibarotene is a small molecule drug that targets the RARα protein. It is primarily used in the treatment of neoplasms, hemic and lymphatic diseases. The drug has been approved for several indications, including acute promyelocytic leukemia, myelodysplastic syndromes, RARA positive myelodysplastic syndrome, and acute myeloid leukemia.
Tamibarotene was developed by Nippon Shinyaku Co., Ltd., a pharmaceutical company based in Japan. It received its first approval in Japan in April 2005. The drug has also undergone regulatory processes such as Fast Track designation and Orphan Drug status.
As a small molecule drug, Tamibarotene is designed to interact with the RARα protein, which plays a role in the development and differentiation of cells. By targeting this protein, the drug aims to inhibit the growth of cancer cells and promote the maturation of abnormal cells.
The approval of Tamibarotene for multiple indications highlights its potential in the treatment of various hematological malignancies. Acute promyelocytic leukemia, a subtype of acute myeloid leukemia, is characterized by the fusion of two genes, including the RARA gene. Tamibarotene's ability to target RARα makes it a suitable treatment option for this specific subtype.
The drug's approval in Japan in 2005 suggests that it has been available to patients in that country for over a decade. The Fast Track designation and Orphan Drug status indicate that Tamibarotene may have shown promising results in clinical trials and addresses an unmet medical need.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for tamibarotene: RARα agonists
From a biomedical perspective, RARα agonists are a type of medication that activate the retinoic acid receptor alpha (RARα). RARα is a nuclear receptor that plays a crucial role in regulating gene expression and cell differentiation. By binding to RARα, these agonists can modulate the transcription of target genes involved in various biological processes.
RARα agonists have shown potential therapeutic applications in the treatment of certain cancers, particularly acute promyelocytic leukemia (APL). In APL, a fusion protein called PML-RARα is formed, which disrupts normal cell differentiation. RARα agonists can bind to this fusion protein and promote the differentiation of leukemia cells, leading to their elimination.
Additionally, RARα agonists have been investigated for their potential in treating other diseases, such as neurodegenerative disorders and metabolic diseases. By targeting RARα, these agonists can modulate gene expression and cellular processes involved in these conditions.
It is important to note that the specific mechanism of action and therapeutic applications of RARα agonists may vary depending on the specific compound being used. Further research and clinical trials are needed to fully understand their potential benefits and limitations in different biomedical contexts.
Drug Target R&D Trends for tamibarotene
RARα, or retinoic acid receptor alpha, is a crucial protein found in the human body that plays a significant role in various physiological processes. As a member of the nuclear receptor superfamily, RARα acts as a transcription factor, regulating gene expression in response to retinoic acid, a derivative of vitamin A. RARα is involved in embryonic development, cell differentiation, and tissue homeostasis. Dysregulation of RARα has been linked to several diseases, including cancer and developmental disorders. Understanding the role of RARα is essential for the development of targeted therapies and drugs that can modulate its activity for therapeutic purposes.
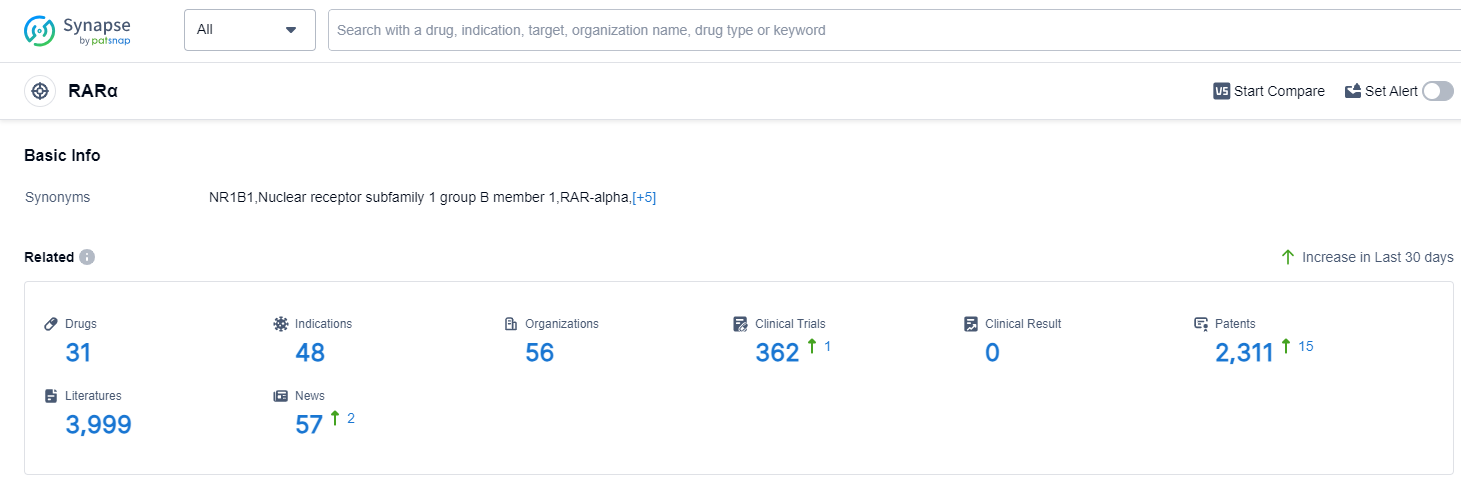
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 31 RARα drugs worldwide, from 56 organizations, covering 48 indications, and conducting 362 clinical trials.
The analysis of the current competitive landscape of target RARα reveals that Bausch Health Cos., Inc. is the leading company with the highest stage of development. The indications for drugs targeting RARα cover a wide range of diseases and conditions, indicating the potential for diverse therapeutic applications. Small molecule drugs are the most rapidly progressing drug type under the current target. The United States and China are the countries/locations with the highest development progress, with China showing significant growth in terms of approved drugs and ongoing research. Overall, the target RARα presents a competitive landscape with multiple companies, diverse indications, and a focus on small molecule drugs. The future development of target RARα holds promise for advancements in the treatment of various diseases and conditions.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Tamibarotene is a small molecule drug developed by Nippon Shinyaku Co., Ltd. It targets the RARα protein and is approved for the treatment of various hematological malignancies, including acute promyelocytic leukemia and myelodysplastic syndromes. Its approval in Japan in 2005 and regulatory designations such as Fast Track and Orphan Drug suggest its potential as a valuable treatment option in the field of biomedicine.