U.S. FDA Approves BMS Reblozyl® as First-Line Treatment of Anemia in Adults with Lower-Risk MDS
Bristol Myers Squibb disclosed that the U.S. Food and Drug Administration (FDA) granted approval to Reblozyl® (luspatercept-aamt) as a therapy for anemia in ESA-naïve (patients who have not previously used erythropoiesis stimulating agents) adult individuals with very-low to intermediate-risk myelodysplastic syndromes (MDS) who may need regular red blood cell (RBC) transfusions. The approval is rooted in interim results from the critical Phase 3 COMMANDS trial which pointed out Reblozyl's superior efficacy in achieving RBC transfusion independence (RBC-TI) as well as an increase in hemoglobin (Hb), compared to epoetin alfa, regardless of the ring sideroblast status. This supports Reblozyl's potential to tackle chronic anemia early in the treatment process for a wider patient demographic.
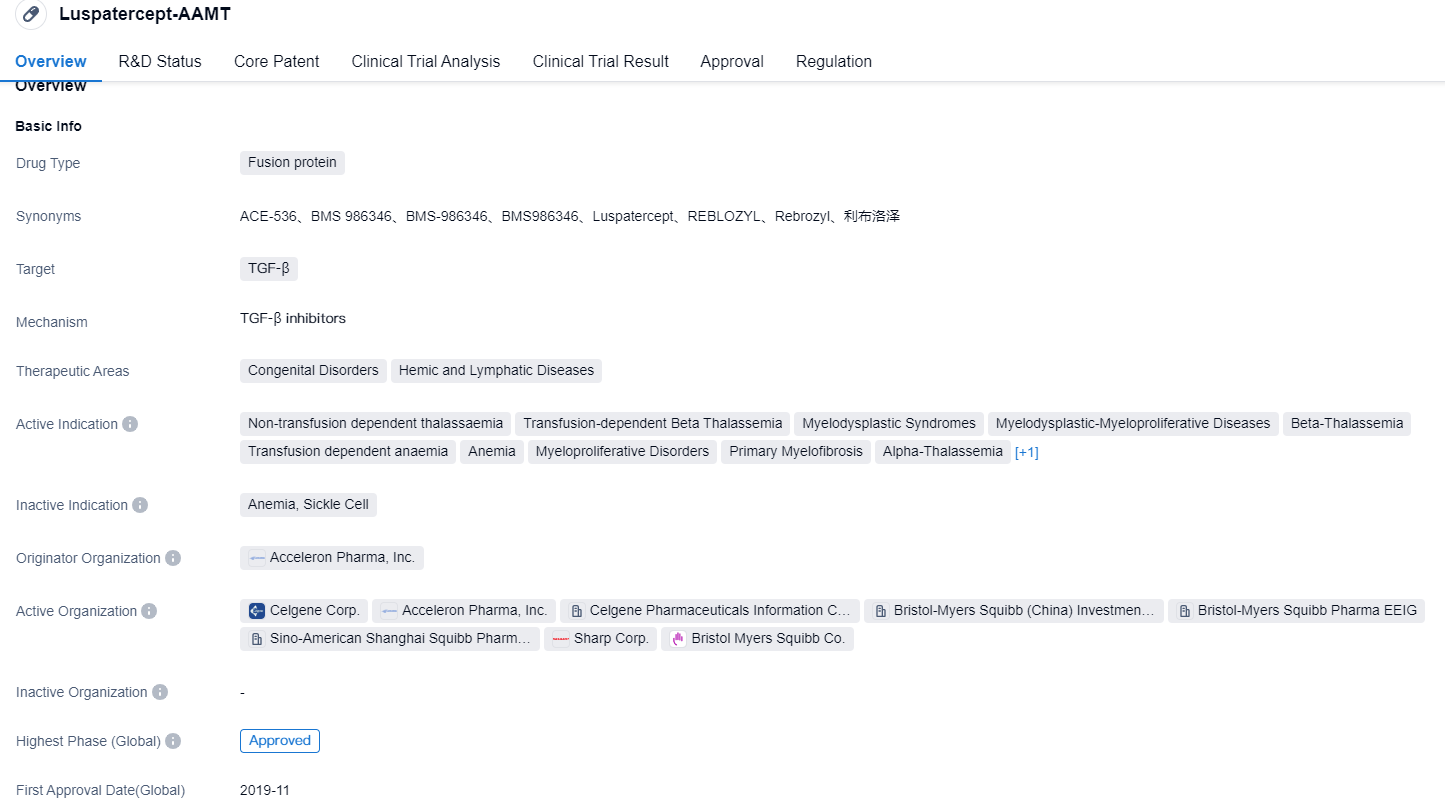
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
According to Guillermo Garcia-Manero, M.D., the primary investigator and head of the Myelodysplastic Syndromes Department at The University of Texas MD Anderson Cancer Center, the current standard therapies including ESAs, offer minimal relief for patients with lower-risk MDS. Data from the COMMANDS study indicates that nearly double the patients who used Reblozyl achieved transfusion independence of a minimum of 12 weeks coupled with an upsurge in hemoglobin in contrast to epoetin alfa. The endorsement is a crucial step forward for patients with lower-risk MDS.
In the Phase 3 COMMANDS experiment, it was noted that 58.5% of patients administered with Reblozyl achieved the primary result of RBC-TI for at least 12 weeks with a mean Hb increase of at least 1.5 g/dL during the first 24 weeks, compared to the 31.2% of patients who were treated with epoetin alfa. The most common adverse reactions included diarrhea, exhaustion, hypertension, peripheral edema, nausea, and dyspnea.
Wendy Short-Bartie, senior VP and general manager, U.S. Hematology and Cell Therapy at Bristol Myers Squibb remarked that the newly expanded approval of Reblozyl aligns with their commitment to assist MDS patients suffering from anemia by offering a long-lasting and efficacious treatment alternative.
Tracey Iraca, executive director, MDS Foundation added that the majority of MDS patients suffer from chronic anemia and require RBC transfusions.
Results from the COMMANDS study were displayed at the American Society of Clinical Oncology Annual Meeting Press Program in June and were concurrently published in The Lancet. Reblozyl is being developed and sold globally as part of a collaboration with Merck starting from November 2021.
The COMMANDS study is an open-label, random Phase 3 study evaluating the efficacy and safety of Reblozyl against epoetin alfa in the treatment of anemia in very low-, low- or intermediate-risk (IPSS-R) MDS patients who are RBC transfusion-dependent and were ESA-naïve. The primary endpoint aimed at in this study is RBC-TI for 12 weeks with a mean hemoglobin (Hb) increase ≥1.5 g/dL. Other important points include HI-E of at least 8 weeks during weeks 1-24 of the study, RBC-TI ≥12 weeks and RBC-TI for 24 weeks.
As of the scheduled interim analysis on October 31, 2022, 147 test subjects who were fit for the study were given Reblozyl and 154 were administered with epoetin alfa, with the median periods of the treatment being 41.6 and 27 weeks, consequently. The Lancet published the results which revealed:
• 58.5% (n=86) of individuals taking Reblozyl as opposed to 31.2% (n=48) receiving epoetin alfa reached the principal endpoint of RBC-TI lasting not less than 12 weeks with an associated mean Hb increment of at least 1.5 g/dL within the initial 24 weeks (p<0.0001).
• 74.1% (n=109) of patients on Reblozyl compared to 51.3% (n=79) on epoetin alfa achieved an HI-E increment of at least 8 weeks (p<0.0001).
• A duration of 24 weeks or more was attained within the first 24 weeks of treatment by 47.6% (n=70) of Reblozyl patients vs. 29.2% (n=45) of those on epoetin alfa (p=0.0012).
• RBC-TI of not less than 12 weeks was met by 66.7% (n=98) of Reblozyl subjects and 46.1% (n=71) of those on epoetin alfa (p=0.0003).
Reblozyl-treated patients displayed persistent responses, with nearly 2.5 years (approx. 126.6 weeks from week 1 to the end of treatment) of median RBC-TI ≥12 weeks. The most common (>10%) side effects were fatigue, hypertension, diarrhea, dyspnea, nausea, and edema.
About Reblozyl® (luspatercept-aamt)
Reblozyl, the first medication of its kind, encourages the late-stage maturation of red blood cells. Merck acquired Acceleron Pharma, Inc. in November 2021, and accordingly, the global collaboration and North American heed for Reblozyl was co-promoted. Milestones and royalties were collected as part of the partnership, and, with this authorization, Reblozyl is earmarked in the U.S. for treating:
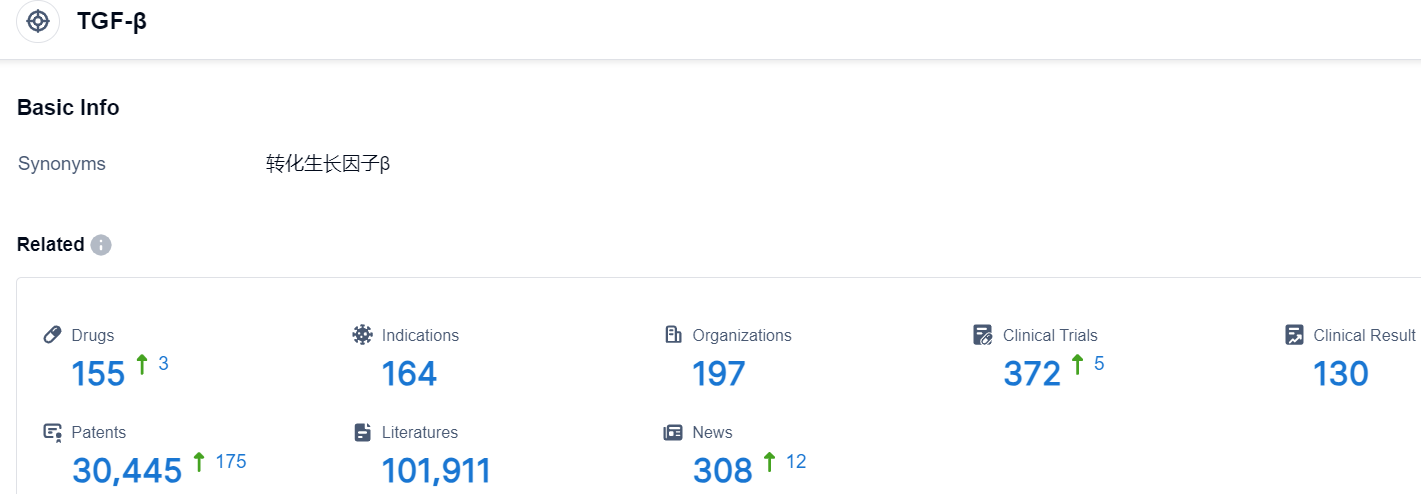
Click on the image below for direct access to the latest R&D progress on TGF-β target drugs, indications, research institutions, clinical trials, and more. as of August 30, 2023, there are 155 drugs under research for the TGF-β target, involving 164 indications, 197 research institutions, and related clinical trials amounting to 372, with as many as 30445 patents. Bristol Myers Squibb has a singular mission - To change lives via scientific breakthroughs. The aim of their cancer research is to produce treatments ensuring improved, healthier existence for each patient and offer possible cures. Leveraging a rich legacy across diverse cancer types that significantly bettered survival rates, Bristol Myers Squibb's researchers are pioneering new realms in personalized medicine, and implementing innovative digital platforms to transform data into knowledge that enhances their acuity. Their profound scientific knowledge, advanced ability and innovative platforms enable an all-sides look at cancer. Cancer's tenacious hold on numerous life facets is well acknowledged by Bristol Myers Squibb who's devoted to addressing every aspect of care, from diagnosis to getting better. As a pioneering entity in cancer care, Bristol Myers Squibb is committed to ensuring everyone with cancer has a brighter future.