Using Synapse for Your Promethazine Research: An Easy Guide
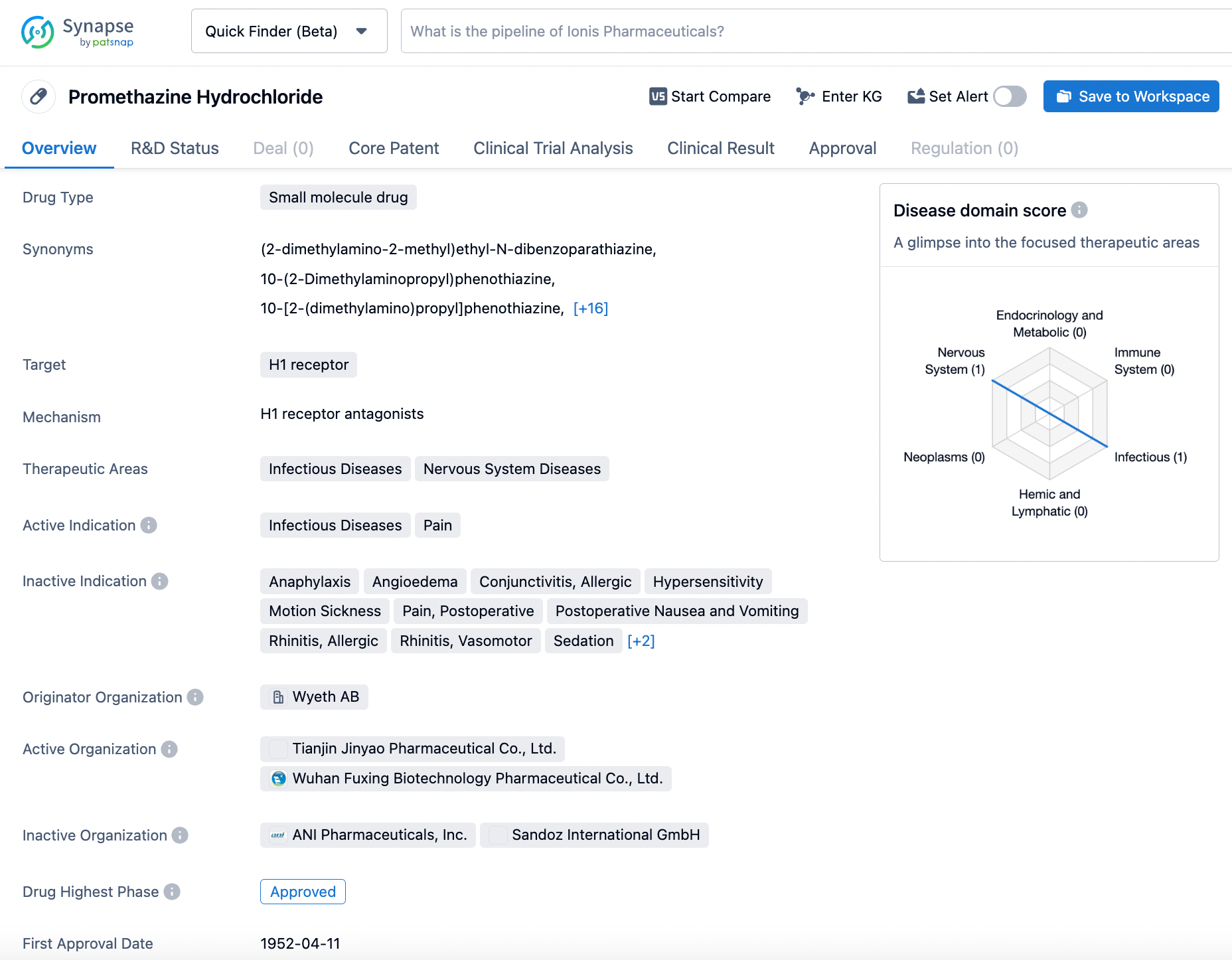
Promethazine, also known by the trade name PHENERGAN®, is a drug that works as an H1 receptor antagonist. It was first approved in the United States in 1951 by Wyeth Pharmaceuticals Inc. Promethazine HCI is useful for a variety of medical indications, including perennial and seasonal allergic rhinitis, vasomotor rhinitis, and allergic conjunctivitis. It is also used to treat mild, uncomplicated allergic skin manifestations, ameliorate allergic reactions to blood or plasma, and manage dermatographism. Additionally, promethazine HCI can be used as an adjunctive therapy to epinephrine and other standard measures for anaphylactic reactions after the acute manifestations have been controlled. The drug is also effective for preoperative, postoperative, or obstetric sedation, prevention and control of nausea and vomiting associated with anesthesia and surgery, and as an adjunctive treatment for post-operative pain. Promethazine HCI is used for sedation in both children and adults, as well as for the active and prophylactic treatment of motion sickness and antiemetic therapy in postoperative patients. Click on the image below to begin the exploration journey of Promethazine through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!