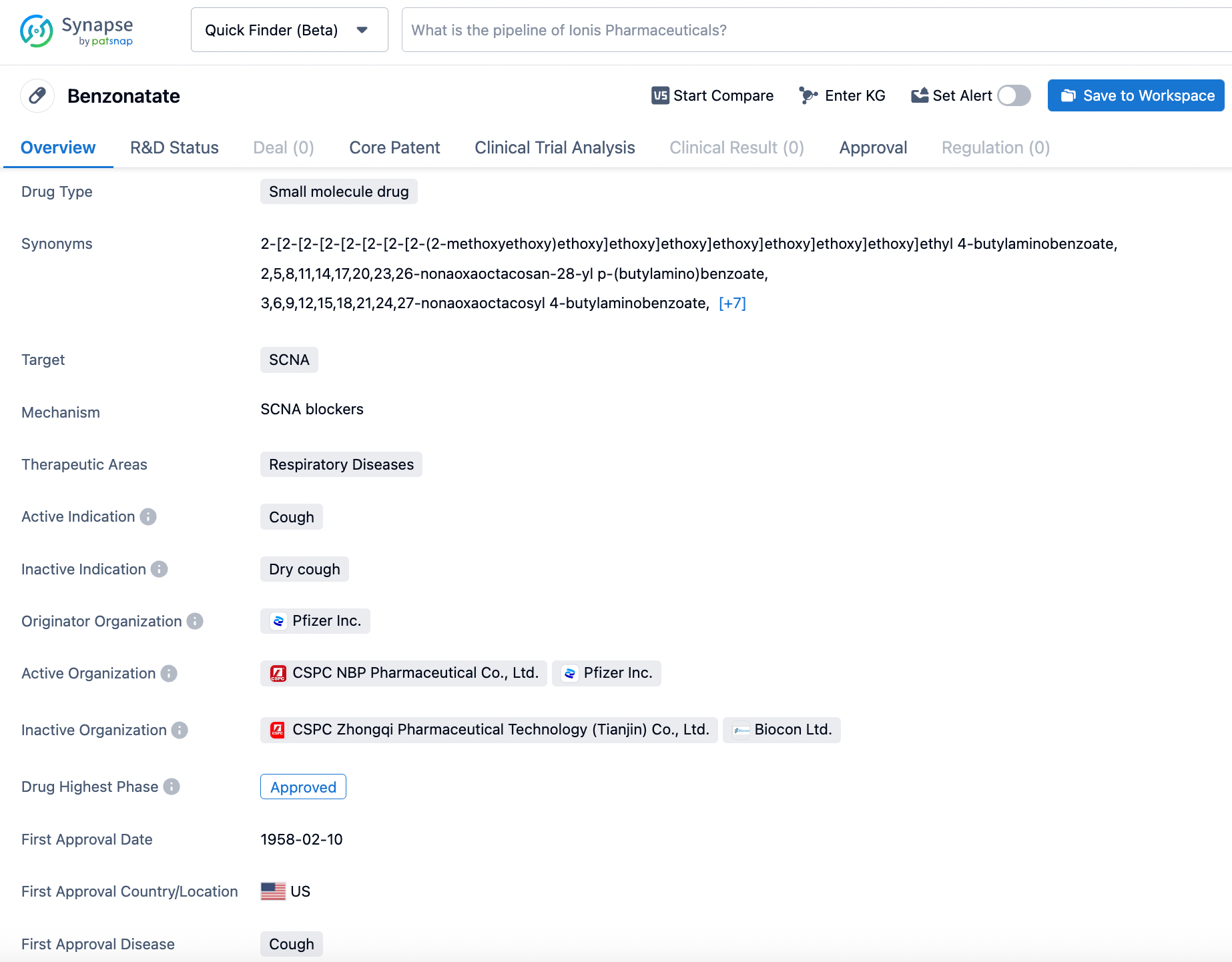
Master Benzonatate Search on Synapse
TESSALON® (Benzonatate) is an FDA-approved non-narcotic oral antitussive medication manufactured by Pfizer Inc. It was first approved in the United States in 1958 for the symptomatic relief of cough. TESSALON acts as a sodium channel blocker, anesthetizing the stretch receptors in the respiratory passages, lungs, and pleura to dampen their activity and reduce the cough reflex. Its effect starts within 15-20 minutes and can last between 3-8 hours. TESSALON has no inhibitory effect on the respiratory center when used as recommended. The active ingredient, 2, 5, 8, 11, 14, 17, 20, 23, 26-nonaoxaoctacosan-28-yl p-(butylamino) benzoate, has a molecular weight of 603.7. TESSALON is solely indicated for the relief of cough, but it has no known potential for abuse or addiction, unlike narcotic antitussives. Click on the image below to begin the exploration journey of Benzonatate through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!