Ventyx Biosciences Begins Phase 2a Trial Dosing of VTX3232 for Early Parkinson’s Disease
Ventyx Biosciences, Inc. (Nasdaq: VTYX) (“Ventyx”), a biopharmaceutical firm currently in the clinical stages, dedicated to developing innovative oral treatments targeting a wide array of inflammatory diseases that lack adequate medical solutions, announced that the initial patient has been administered a dose in a Phase 2a study of VTX3232 for individuals with early-stage Parkinson’s disease.
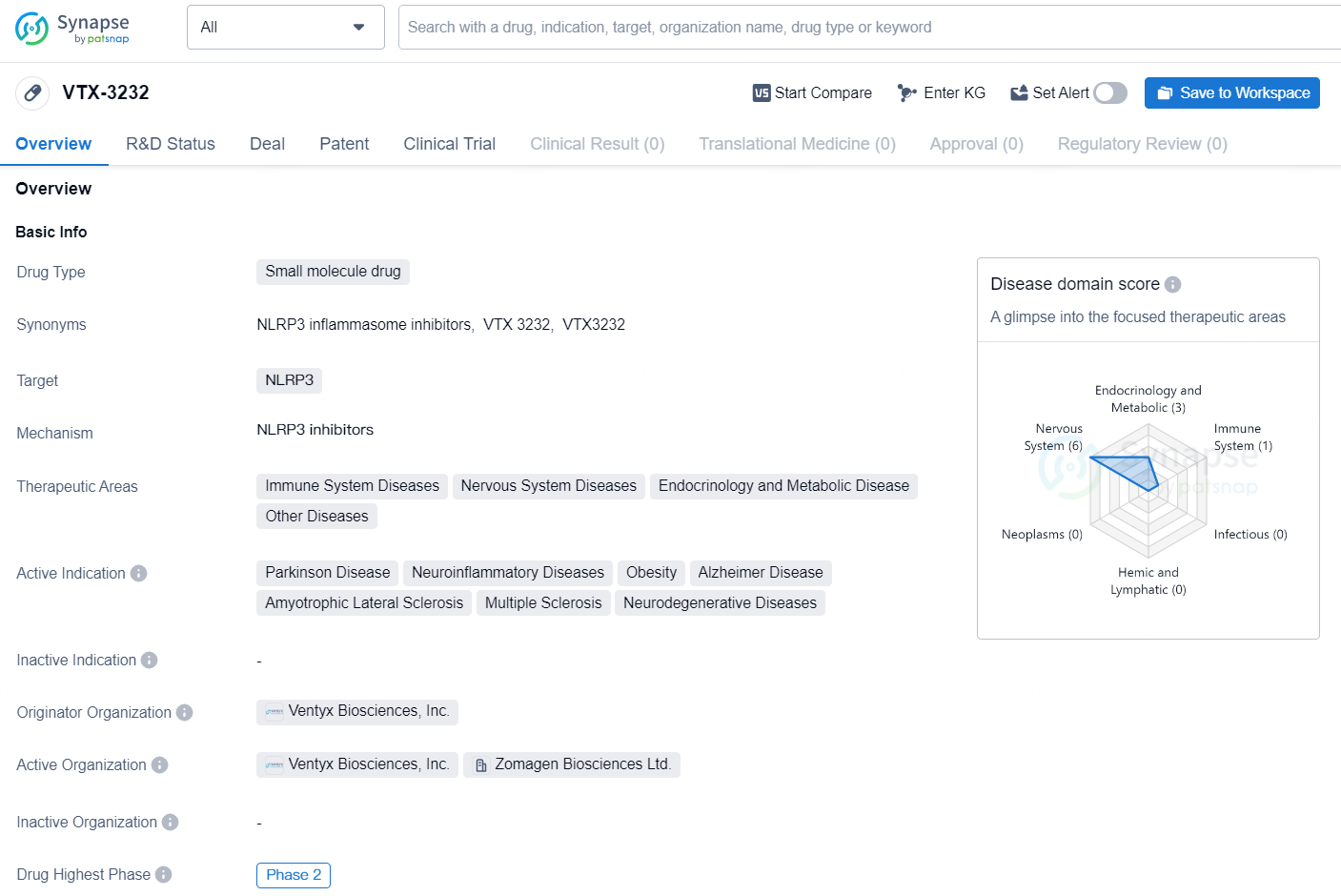
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We are thrilled to begin this clinical study of VTX3232 in individuals with early-stage Parkinson’s disease," commented Mark Forman, MD, PhD, Chief Medical Officer. "The existing body of literature presents a strong mechanistic basis for targeting NLRP3-driven neuroinflammation in Parkinson’s disease. Activation of microglial NLRP3 appears to significantly contribute to the disease's pathogenesis and neurodegeneration. This study aims to assess the effects of VTX3232 on key biomarkers related to the disease and its targets, incorporating exploratory PET neuroimaging to observe the impact of VTX3232 on microglial activation. These assessments may provide early indications of VTX3232’s potential in mitigating Parkinson’s disease pathology by inhibiting NLRP3 in the CNS."
The Phase 2a clinical trial of VTX3232 in early-stage Parkinson’s disease plans to include roughly ten patients over a 28-day open-label treatment period. The primary focus of the trial is safety and tolerability. Additional measures will include pharmacokinetics and relevant biomarkers in both plasma and cerebrospinal fluid. We anticipate releasing the topline findings from this study in 2025.
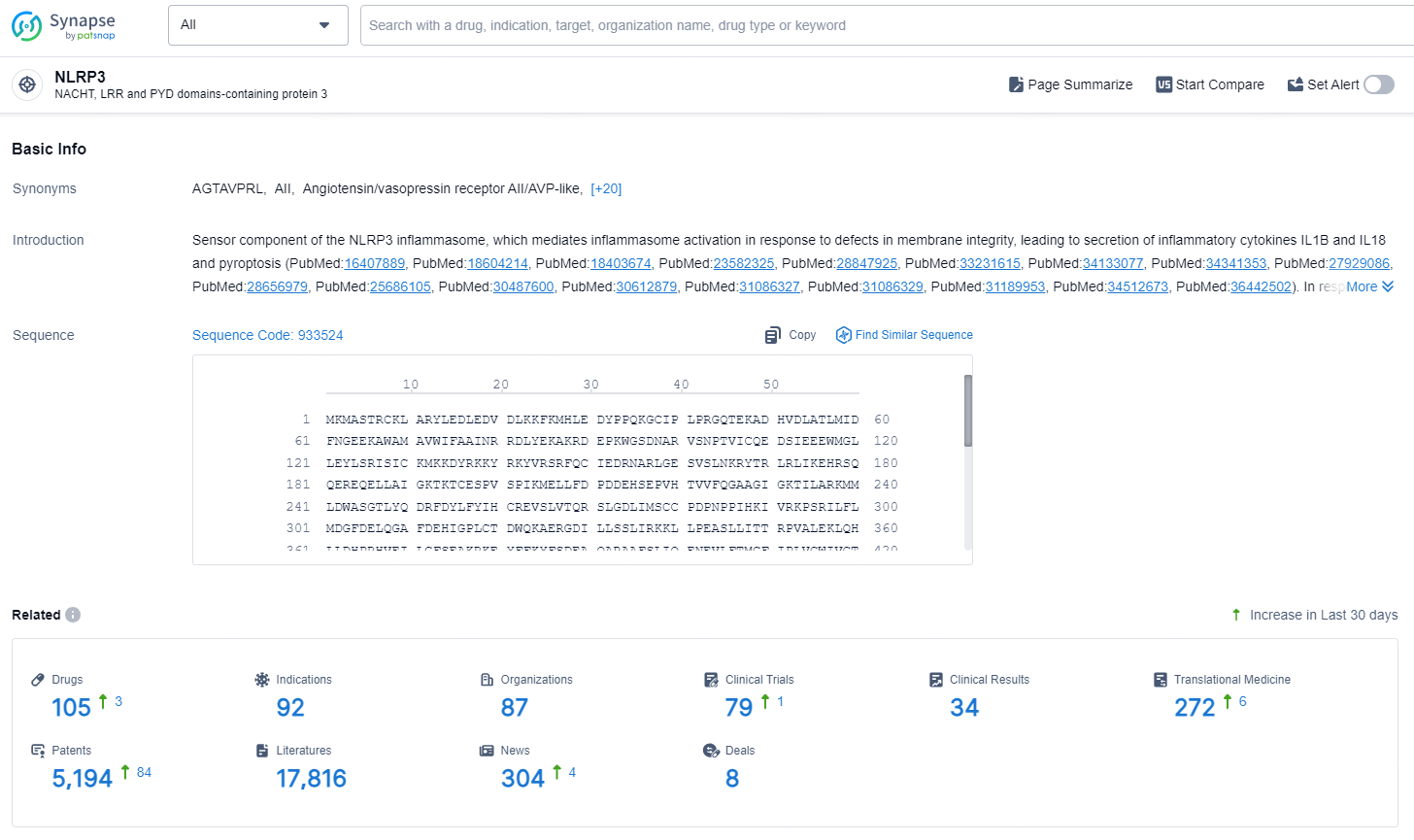
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 9, 2024, there are 105 investigational drug for the NLRP3 targets, including 92 indications, 87 R&D institutions involved, with related clinical trials reaching 79, and as many as 5194 patents.
VTX-3232 is a small molecule drug developed by Ventyx Biosciences, Inc., with NLRP3 as its molecular target. The drug is being investigated for its potential in treating a wide range of therapeutic areas, including immune system diseases, nervous system diseases, endocrinology and metabolic diseases, as well as other diseases. The active indications for VTX-3232 include Parkinson Disease, neuroinflammatory diseases, obesity, Alzheimer Disease, amyotrophic lateral sclerosis, multiple sclerosis, and other neurodegenerative diseases.