Vertex and CRISPR Therapeutics have declared that CASGEVY™ has been officially sanctioned by the FDA to combat sickle cell disease
Vertex Pharmaceuticals Incorporated, in partnership with CRISPR Therapeutics, has shared news that the U.S. Food and Drug Administration (FDA) has given the green light to their innovative treatment, CASGEVY™ (exagamglogene autotemcel [exa-cel]). This groundbreaking therapy, based on the CRISPR/Cas9 gene-editing technology, is now authorized for use in individuals aged 12 and above who are afflicted with sickle cell disease and suffer from repeated vaso-occlusive episodes.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The green light for CASGEVY from the FDA signifies a historic moment, as it becomes the maiden therapy with the foundation of CRISPR gene-editing to secure clearance in the United States. Around 16,000 SCD sufferers now have the prospect of receiving a groundbreaking, single-application treatment that aspires to serve as a sustainable corrective option for their condition, seeking to abolish the occurrence of significant VOCs and the need for subsequent hospitalizations.
Vertex's senior leadership, Reshma Kewalramani, M.D., the CEO and President, expressed her profound appreciation to the participants and researchers whose faith in the initiative has been critical to achieving this significant milestone.
Samarth Kulkarni, Ph.D., CEO and Chairman of CRISPR Therapeutics, reflected on the origin of the organization with ambitions to develop pioneering therapies utilizing CRISPR technology. The endorsement of the first CRISPR-based medicine in the U.S. is seen as an exhilarating step forward, instilling pride and honor throughout the team.
Highlighting the extraordinary journey, Stephan Grupp, M.D., Ph.D., who oversees the Cellular Therapy and Transplant Section and directs the Kelly Center for Cancer Immunotherapy at the Children's Hospital of Philadelphia, and chairs the Steering Committee for the CLIMB-121 clinical program, accentuated CASGEVY's potential to revolutionize treatment paradigms for patients. He anticipates ongoing endeavors to expand access to this innovative therapy nationwide.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
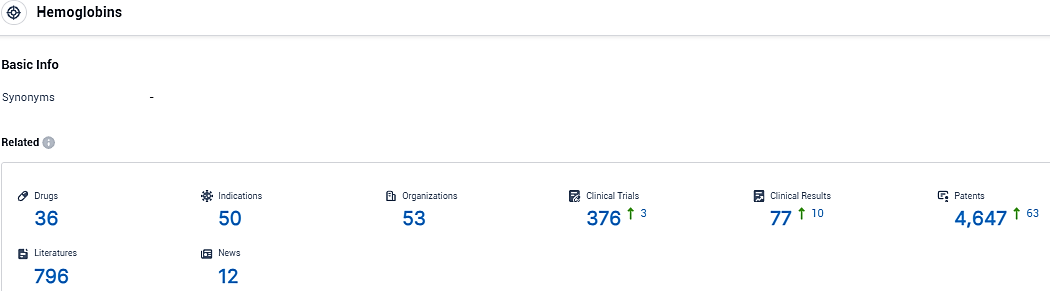
According to the data provided by the Synapse Database, As of December 15, 2023, there are 36 investigational drugs for the hemoglobin target, including 50 indications, 53 R&D institutions involved, with related clinical trials reaching 376, and as many as 4647 patents.
CASGEVY is intended for one time administration via a hematopoietic stem cell transplant procedure where the patient’s own CD34+ cells are modified to reduce BCL11A expression in erythroid lineage cells, leading to increased fetal hemoglobin production. CASGEVY was granted a conditional marketing authorization in Great Britain by the U.K.