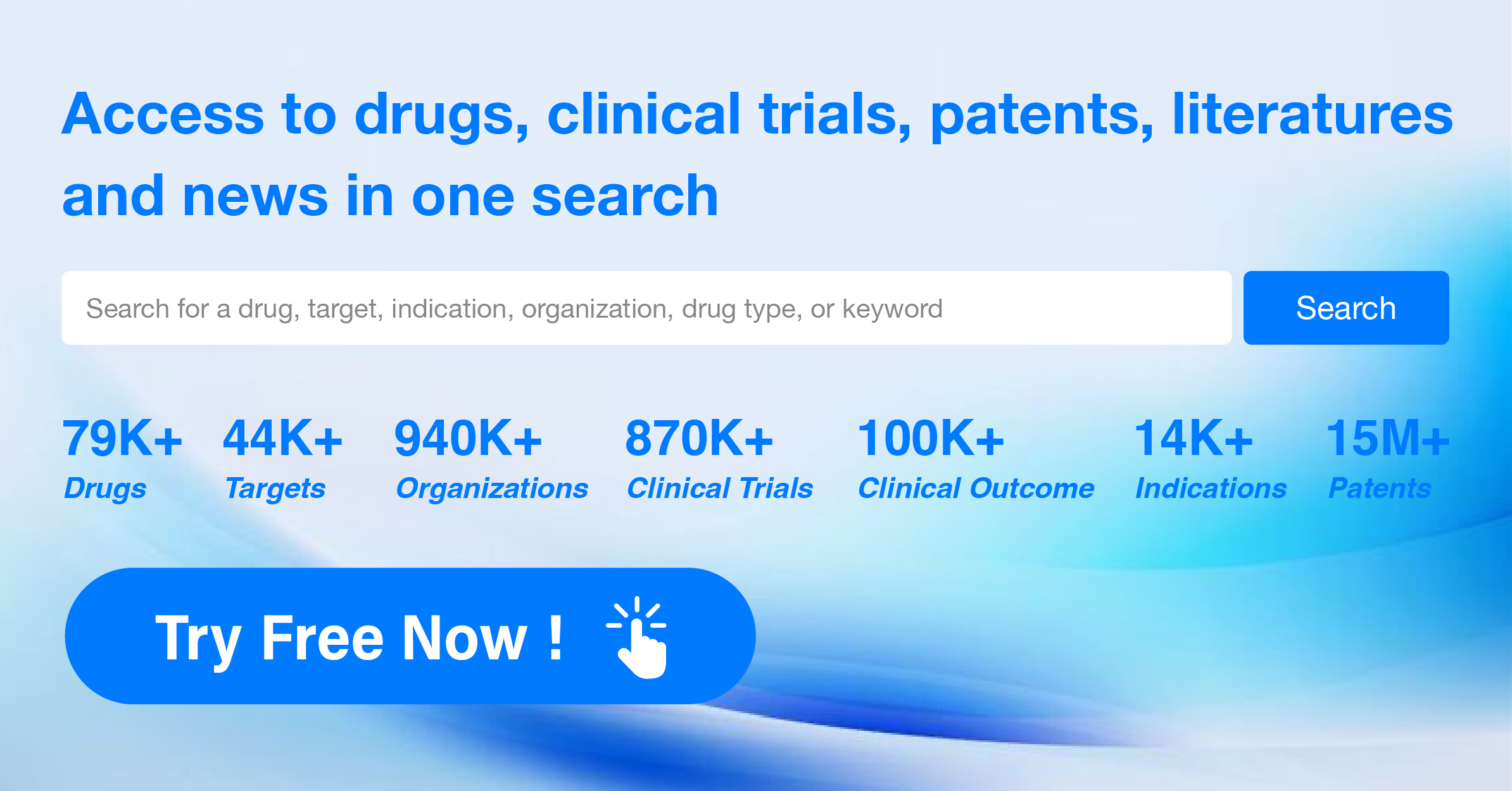
Understanding PD-L1 Inhibitors and Methods to Keep Abreast of Their Recent Developments
Programmed death-ligand 1 (PD-L1) is a protein found on the surface of certain cells in the human body, including immune cells and some cancer cells. Its primary role is to regulate the immune response by interacting with a receptor called PD-1 on T cells. When PD-L1 binds to PD-1, it sends inhibitory signals that suppress the activity of T cells, preventing them from attacking healthy cells. However, some cancer cells exploit this mechanism by overexpressing PD-L1, which helps them evade immune detection and destruction. Targeting the PD-L1/PD-1 interaction with specific drugs, known as immune checkpoint inhibitors, has revolutionized cancer treatment by restoring the immune system's ability to recognize and eliminate cancer cells.
Immunotherapy, particularly PD-1/PD-L1 monoclonal antibodies, has emerged as a new treatment modality and has achieved significant success in cancer treatment. So far, this approach has been applied to the treatment of diseases such as melanoma, cutaneous squamous cell carcinoma (CSCC), recurrent or advanced endometrial cancer, non-small cell lung cancer, and metastatic Merkel cell carcinoma. However, despite its success in medicine and business, PD-1/PD-L1 monoclonal antibodies have some limitations. Firstly, due to poor diffusion and penetration, clinical efficacy is limited to a small number of patients. Secondly, the pharmacokinetic properties of monoclonal antibodies lead to immunogenicity and toxicity, resulting in serious immune-related adverse events (irAEs). In addition, the manufacturing cost of monoclonal antibodies is high, which will increase the economic burden on patients. Therefore, using small molecule inhibitors to block PD-1/PD-L1 interactions may be another way to activate the innate immune system to fight tumors. Small molecule inhibitors have several advantages, such as oral bioavailability, increased tumor penetration, proximity to intracellular targets, and lower production costs, which may be advantages over antibody-based treatments. Small molecule inhibitors can be used as a single treatment or in combination with other drugs including antibodies, and may provide important supplements or potential synergistic effects in immunotherapy. Since BMS released the first PD-L1 small molecule patent, the World Intellectual Property Organization has issued more than 100 patents. In addition, different biological detection methods have been developed to explore the in vitro and in vivo activity of PD-L1 inhibitors, such as Cisbio’s PD-1/PD-L1 assay kit and Promega’s cellular PD-1/PD-L1 assay kit. In addition, the small molecule co-crystal structure of compound 10 with PD-L1 protein has been published, which lays a good foundation for better rational design of effective PD-L1 inhibitors. The key to the rational discovery of small molecule antagonist drugs is to find a pharmacophore model. Based on the structure and activity of known PD-L1 inhibitors, two pharmacophore models have been summarized. Five compounds have shown good preclinical results and have entered clinical trials. However, compared with the development of PD-1/PD-L1 antibodies, the development of PD-L1 small molecules is still in its infancy. It is unclear whether these compounds can show as good clinical efficacy as antibodies. Compared with the large number of small molecules disclosed in patents, the number of molecules that have entered clinical trials is relatively small. The discovery of inhibitors that rely solely on protein analysis (such as HTRF) has certain limitations. Many compounds do indeed lack clear translation of biological activity in more complex cellular environments. Some inhibitors bind significantly weaker to mouse PD-L1 than to human PD-L1. In other words, it is necessary to analyze the identified inhibitors not only for binding to humans, but also for binding to mouse PD-L1, in order to minimize the risk of non-specific and off-target effects in preclinical studies in mouse models. Therefore, it is still necessary to design better PD-L1 small molecule inhibitors for clinical research, study their safety and effectiveness, and accelerate their approval.
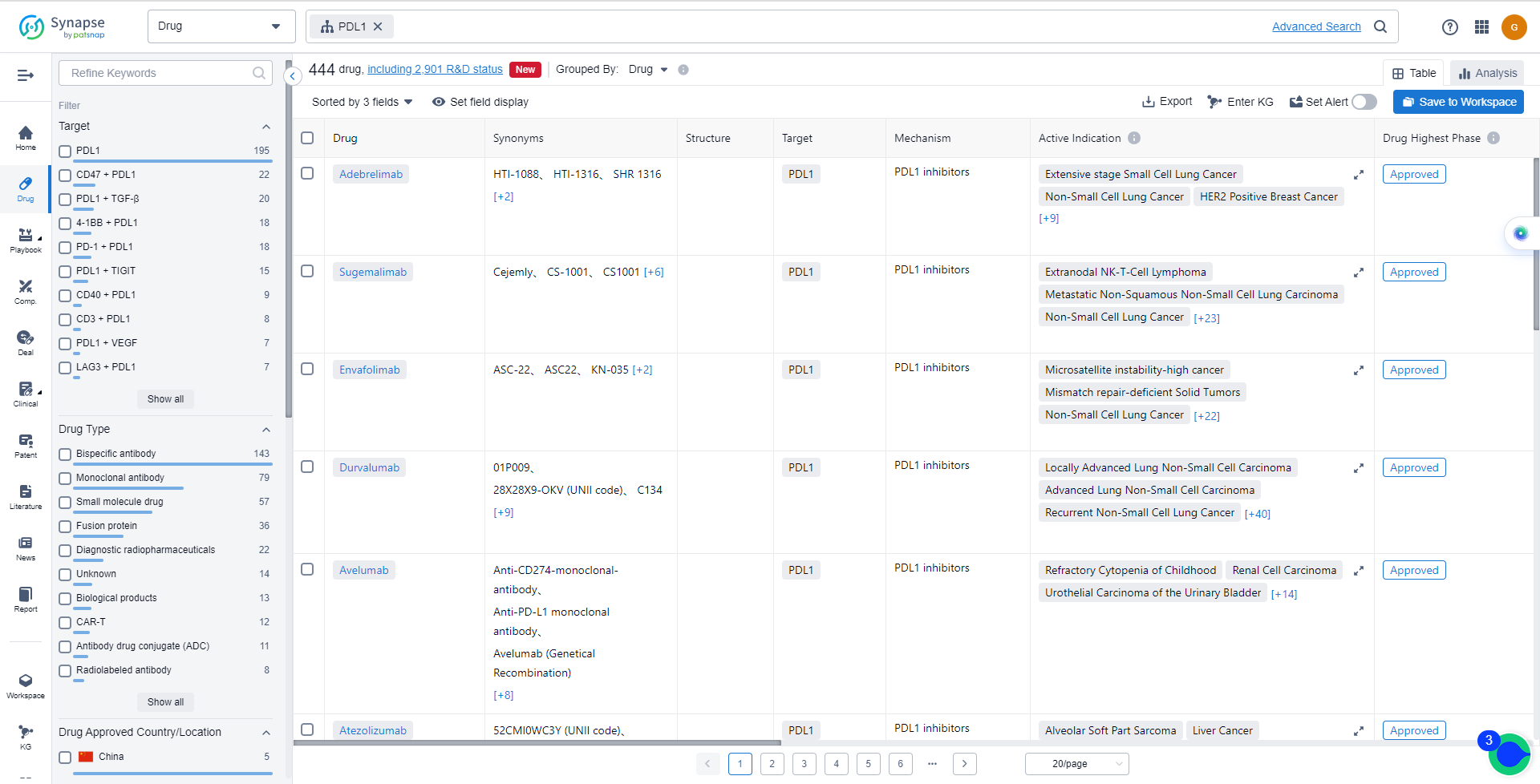
The drug types progressing most rapidly under the target PD-L1 include Monoclonal antibody, Bispecific antibody, and Fusion protein. Biosimilars, such as Monoclonal antibody and Bispecific antibody, indicate intense competition around the innovative drug.
China, the United States, and the European Union are the countries/locations developing fastest under the target PD-L1, with China showing significant progress in drug development. This suggests a promising future for the target PD-L1 in terms of competitive landscape and future development.
Overall, the analysis highlights the active research and development efforts in the pharmaceutical industry for the target PD-L1, with multiple companies, indications, drug types, and countries/locations involved.
How do they work?
From a biomedical perspective, PD-L1 inhibitors are a type of medication used in cancer immunotherapy. PD-L1 stands for programmed death-ligand 1, which is a protein found on the surface of certain cancer cells. PD-L1 interacts with another protein called PD1 on immune cells, leading to suppression of the immune response against cancer cells. PD-L1 inhibitors work by blocking the interaction between PD-L1 and PD1, thereby allowing the immune system to recognize and attack cancer cells more effectively. These inhibitors have shown promising results in the treatment of various cancers, including lung cancer, melanoma, and bladder cancer. However, it is important to note that PD-L1 inhibitors may have side effects and are not suitable for all patients.
List of PD-L1 Inhibitors
The currently marketed PD-L1 inhibitors include:
- Adebrelimab
- Sugemalimab
- Envafolimab
- Durvalumab
- Avelumab
- Atezolizumab
- Benmelstobart
- Cosibelimab
- Socazolimab
- Tagitanlimab
For more information, please click on the image below.
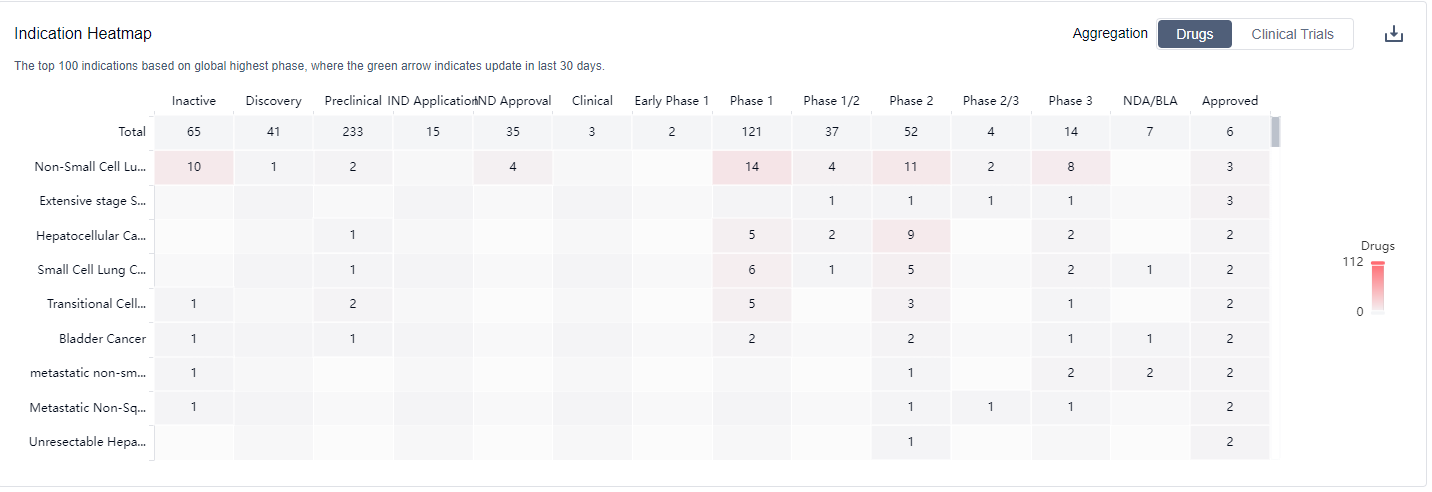
What are PD-L1 inhibitors used for?
PD-L1 inhibitors are under investigation for indications that include including lung cancer, melanoma, and bladder cancer. For more information, please click on the image below to log in and search.
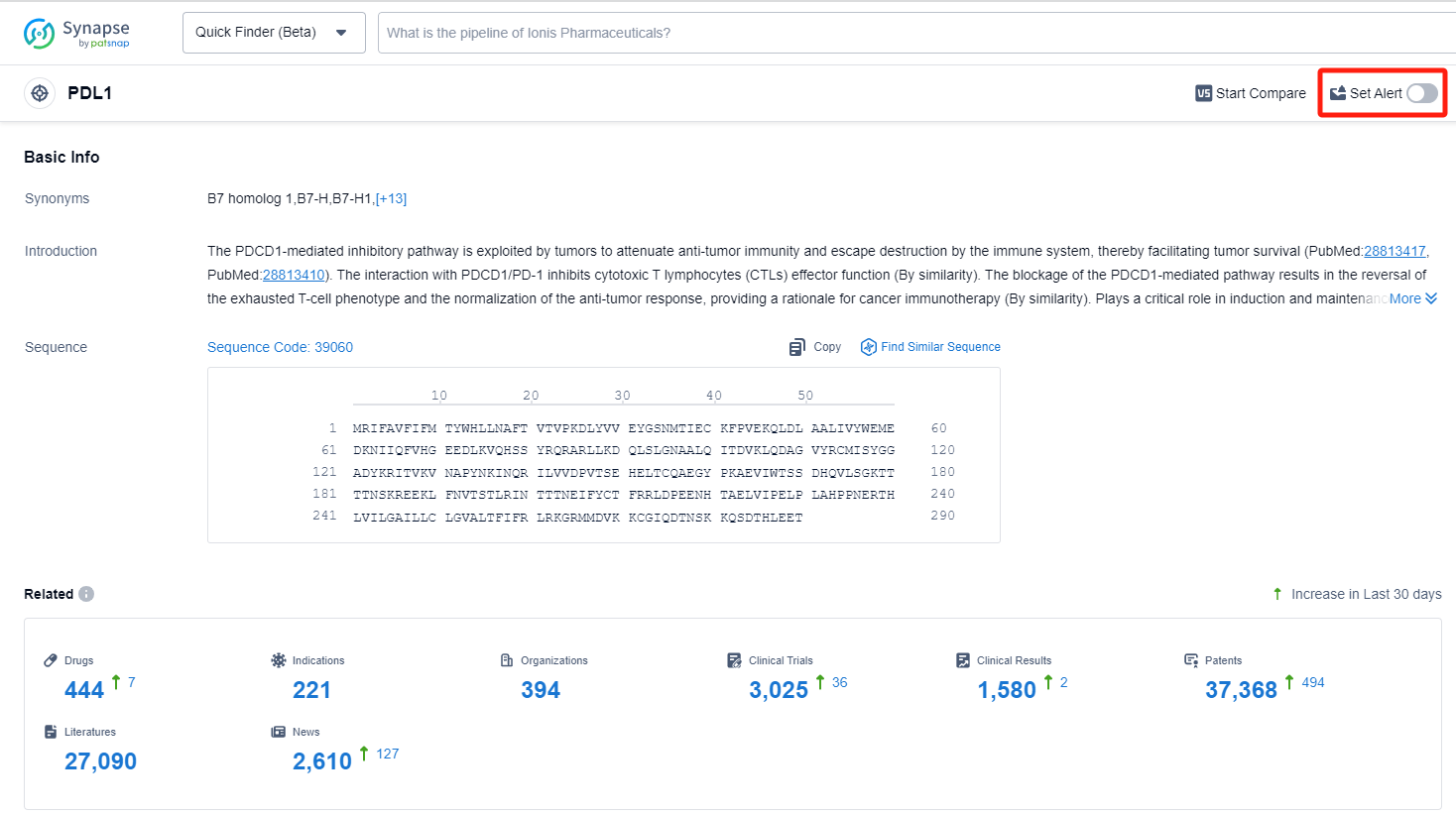
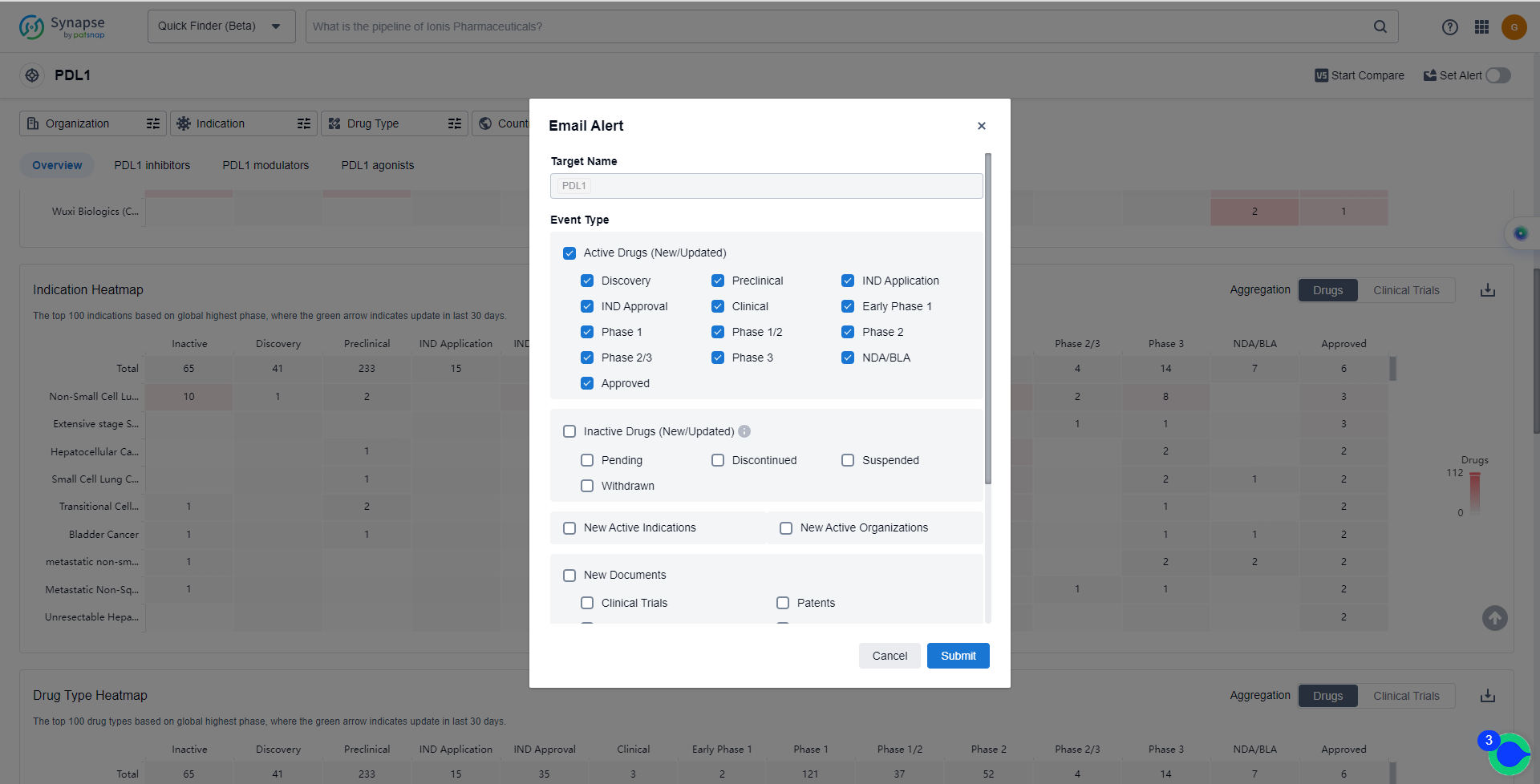
How to obtain the latest development progress of PD-L1 inhibitors?
In the Synapse database, you can keep abreast of the latest research and development advances of PD-L1 inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!