Vidac Pharma Shares Promising Early Phase 2a Trial Results for Rare Skin Cancer Drug, VDA-1102
Vidac Pharma Holdings Plc., an innovative biopharmaceutical firm engaged in the development of new oncology therapeutics, has reported encouraging results from an interim Phase 2a study involving its primary investigational compound, VDA-1102, targeting Mycosis Fungoides. This disease is a variant of Cutaneous T-Cell Lymphoma, which is categorized as a rare "orphan" disease, potentially qualifying for expedited review by regulatory authorities. The emerging clinical data bolster the evidence supporting both the effectiveness and safety profile of Vidac Pharma's unique class of non-toxic small molecule therapeutics in the management of cancerous conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
An examination of half the participant group revealed that the Objective Response Rate was at 56%, encompassing a 22% rate of complete response and 34% of partial response. Responses emerged within the timeframe of 8 to 12 weeks. Such findings are encouraging when juxtaposed with mechlorethamine, a conventional treatment, which only produces a 13% complete response and typically takes about 26 weeks for a median response to manifest. Adverse effects were mostly minor and localized, with only a single case classified as moderate. All participants fully recuperated and showed no disease progression throughout the study's duration, which spanned four months.
Vidac Pharma's CEO, Max Herzberg, commented, “These promising interim outcomes validate Vidac Pharma's innovative strategy of targeting cancer's fundamental mechanisms, demonstrating significant therapeutic promise. Our compounds disrupt irregular cellular metabolism that is a hallmark of cancer, thus stopping the growth of tumors and the development of immune resistance. The implications of these findings suggest a broad application of our treatments for various cancers.”
The provisional data is derived from the majority of the intended subjects—specifically, 9 out of 16—part of a transparent, placebo-controlled assessment examining the impact and safety of the topical remedy VDA-1102, used over a trial period of 12 weeks in grown individuals with relapsed stage-1 MF. Upcoming benchmarks include the complete-group interim outcomes anticipated in the year's second quarter and the definitive findings in the last quarter.
Vidac Pharma is innovating in the sphere of cancer treatment, targeting the aberrant cellular metabolism known as the Warburg effect. This is achieved by inhibiting the Hexokinase 2 (HK2) enzyme from disrupting the mitochondrial VDAC relay channels. Clinical investigations underscore the potential of this method in ending the proliferation of cancerous cells and reinstating their programmed cell demise. Moreover, Vidac Pharma is advancing VDA-1102, a principal compound, into further stages of examination for actinic keratosis, gearing up for an imminent launch of a subsequent Phase 2b trial.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
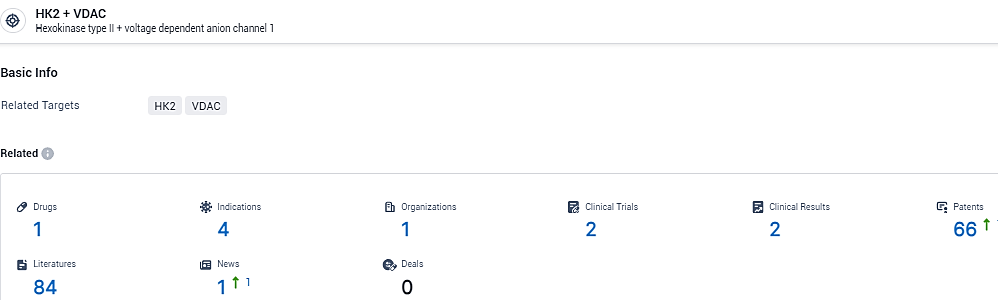
According to the data provided by the Synapse Database, As of February 2, 2024, there are 1 investigational drugs for the HK2 and VDAC target, including 4 indications,1 R&D institutions involved, with related clinical trials reaching 2, and as many as 66 patents.
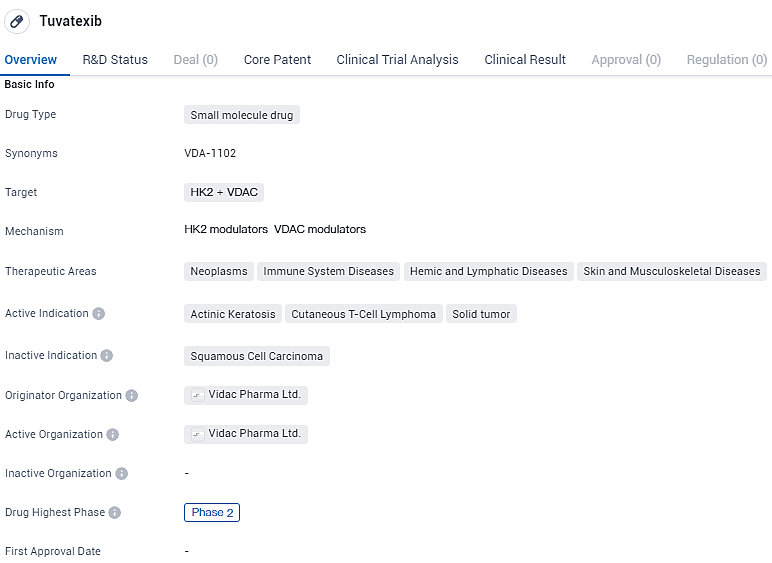
VDA-1102 has reached the highest phase of clinical development, which is Phase 2. This means that the drug has already undergone initial testing in humans to assess its safety and efficacy. Phase 2 trials typically involve a larger number of participants and provide further insights into the drug's effectiveness and potential side effects.