Asieris Presents Early Results of APL-1202 and Tislelizumab Study for Advanced Bladder Tumors at ASCO-GU 2024
Asieris Pharmaceuticals, an international firm focused on biopharmaceutical research, has publicized the debut of the preliminary analysis results from its Phase II study. This study investigates the efficacies of the orally-administered APL-1202 when used in conjunction with the PD-1 inhibiting medication tislelizumab, as a pre-surgical therapeutic approach for those affected by muscle-invasive bladder cancer. The company is renowned for its groundbreaking work in the creation and market introduction of new therapies aimed at combating cancer of the urinary system and associated health conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The preliminary findings from a study were shared through a concise spoken abstract at the 2024 American Society of Clinical Oncology (ASCO) Genitourinary Cancers Symposium. The phase II clinical study has recently finished recruiting participants.
The study's major goal is to assess the therapeutic profile and tolerability of the drug APL-1202 when used in conjunction with tislelizumab versus the use of tislelizumab alone as a pre-surgical treatment for patients with muscle-invasive bladder cancer (MIBC). Individuals who have received an MIBC diagnosis, are scheduled for a radical cystectomy, and cannot use cisplatin or opt out of cisplatin-based preoperative chemotherapy are part of the study's target demographic.
The main measure of success for the study is the rate of pathological complete response (pCR), which is determined when no residual cancer tissue is detected in the bladder or lymph nodes, as verified through histological examination post-radical cystectomy.
Dr. Linda Wu, the Chief Development Officer at Asieris, commented, "Early clinical findings suggest that the synergistic effect of APL-1202 with the PD-1 blocking agent tislelizumab might be a potent therapeutic strategy for use in pre-surgical scenarios for MIBC. We're excited about its potential as an alternative for patients who cannot or choose not to undergo platinum-based treatments."
Currently, APL-1202 is being investigated in two crucial concurrent studies at phase III/pivotal levels. One pivotal phase II study is exploring the drug APL-1202 in combination with therapy delivered directly into the bladder (intravesical chemotherapy) for intermediate and high-risk non-muscle invasive bladder cancer (NMIBC) patients who have not responded to prior chemotherapy. Additionally, a phase III study is examining the effectiveness of APL-1202 as a standalone treatment in patients with intermediate-risk NMIBC who have not been treated before.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
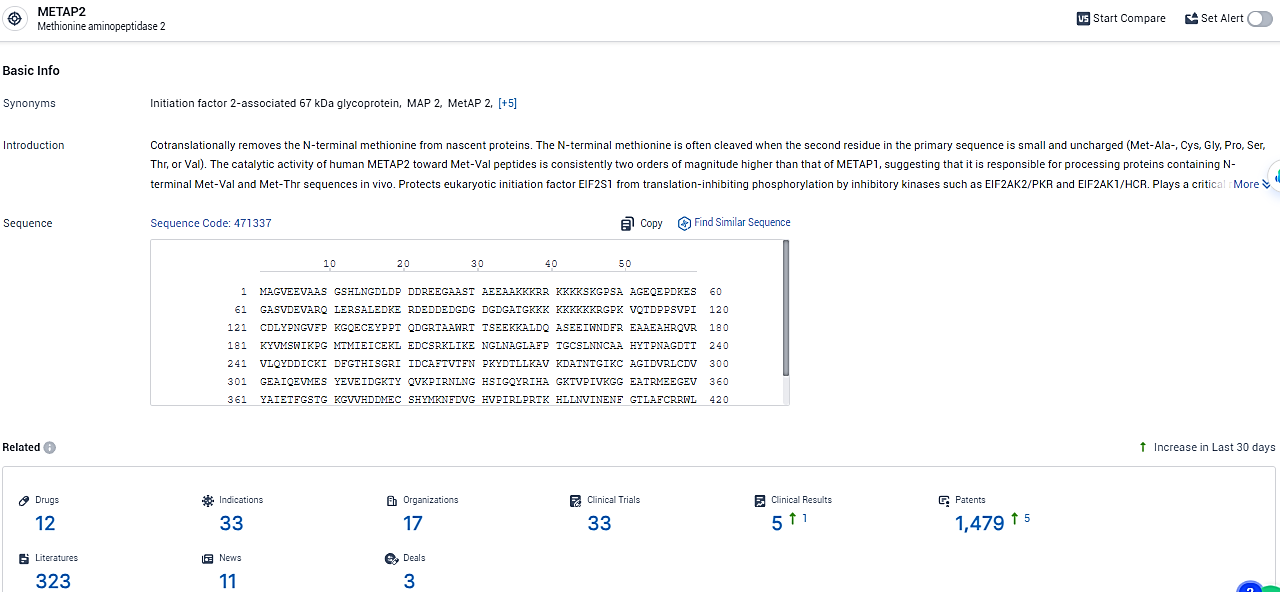
According to the data provided by the Synapse Database, As of February 2, 2024, there are 12 investigational drugs for the METAP2 target, including 33 indications,17 R&D institutions involved, with related clinical trials reaching 33, and as many as 1479 patents.
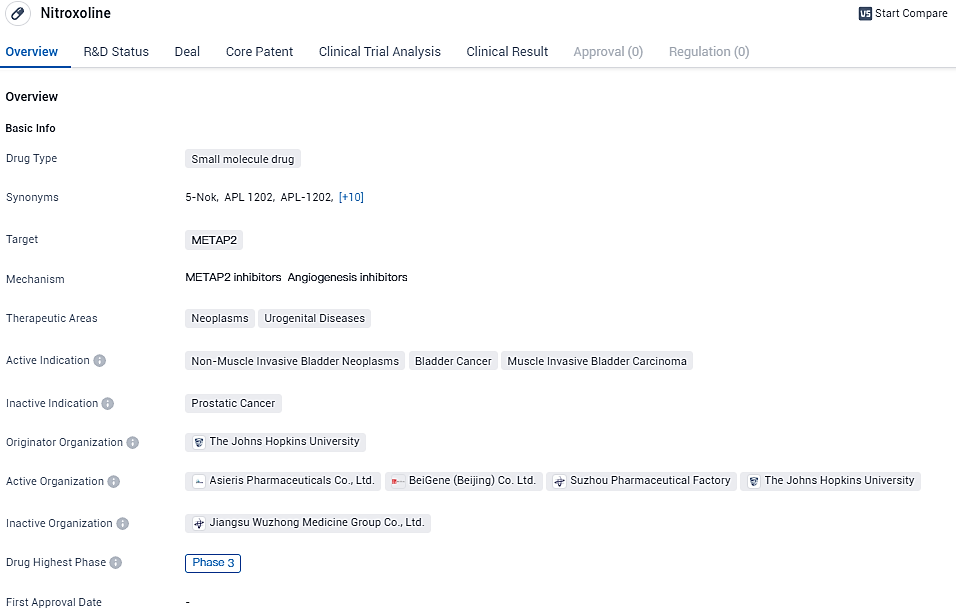
APL-1202 targets METAP2 and is being investigated for its potential therapeutic applications in neoplasms and urogenital diseases. The drug has reached Phase 3 in both global and Chinese clinical trials, indicating promising results in earlier stages of development. If successful, Nitroxoline may become an approved treatment option for various bladder-related conditions.