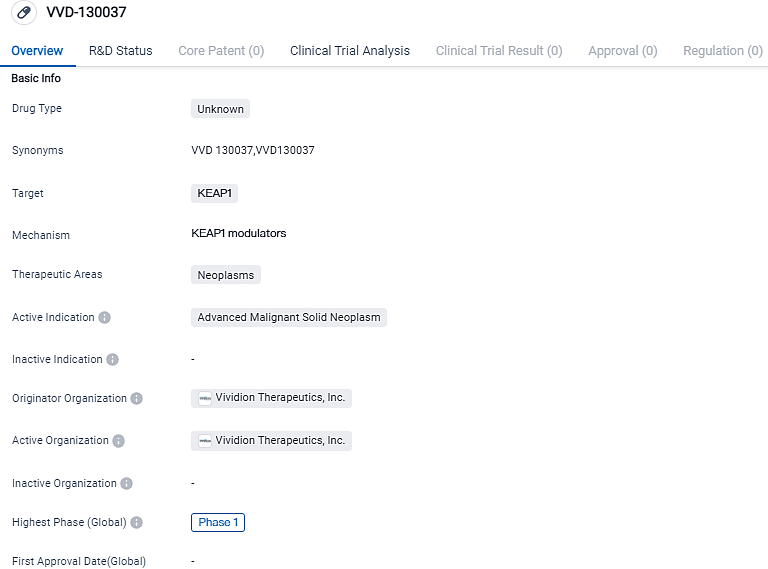
Vividion Therapeutics initiates the phase I clinical trial in advanced solid tumors utilizing KEAP1 activator
Vividion Therapeutics, Inc., confirmed the commencement of patient dosing for a Phase I cancer clinical trial concerning its experimental oral agent, KEAP1 activator VVD-130037. As a biopharmaceutical entity, Vividion leverages pioneering discovery methods to tap into high-value target areas, typically unresponsive to conventional drugs, creating precision treatments for severe cancerous conditions and immune disorders. This trial's onset is a key advancement for the revolutionary chemoproteomics technologies developed at Vividion.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The commencement and dosage in this clinical trial signify a key turning point for Vividion as we progress towards becoming a clinical stage enterprise. It's inspiring to see how far we've come in such a short period with our innovative pipeline for unexplored protein targets, which are crucial in oncology and immunology diseases,” stated Aleksandra Rizo M.D., Ph.D., CEO of Vividion. “Our motivation for the future is high as we aim to bring numerous programs to the clinical front from 2023 onwards."
Vividion’s proprietary chemoproteomic approach enables the firm to uncover the potential of traditionally untapped target biology. This approach results in precise therapies for cancer and immune disorders and has proven successful since Vividion’s acquisition by Bayer in August 2021. This acquisition has enabled Vividion to work independently, implying a win-win situation – it maintains its creativity and entrepreneurial spirit while utilizing Bayer's proficiency in small molecule development, international capabilities and financial strength.
"We're thrilled to kick start and administer Vividion’s primary drug in clinical trials stemming from our chemoproteomic platform," Jenna Goldberg, M.D., CMO of Vividion, expressed. "This first-of-its-kind clinical candidate intends to target cancers activating the KEAP1-NRF2 pathway which could revolutionize cancer treatment."
Multiple innovative biology schemes are moving toward the clinic in the company, with over a dozen similar pipeline opportunities popping up in early discovery in oncology and immunology fields. The Phase I clinical trial will look into the safety, pharmacokinetics, and pharmacodynamics, and initial efficiency of VVD-130037 in patients with advanced solid tumors. Trial participants must have histologically verified metastatic or unremovable solid tumor, who will then receive rising dosages of VVD-130037 orally, once daily in 21-day treatment courses.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
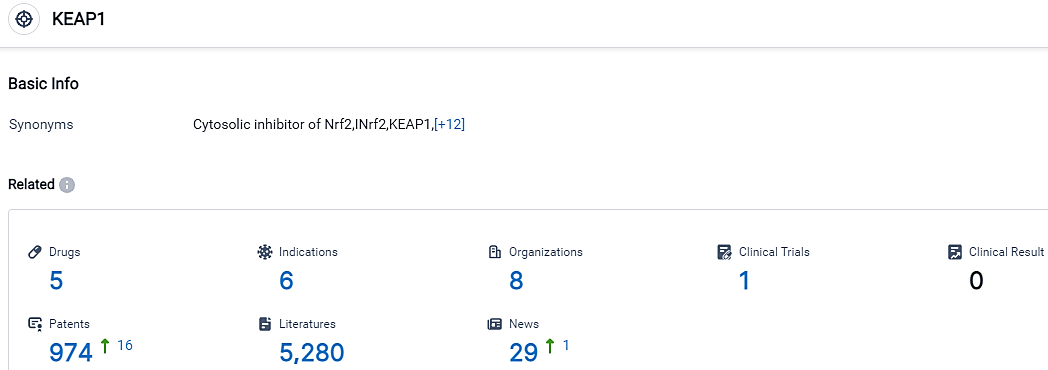
According to the data provided by the Synapse Database, As of September 20, 2023, there are 5 investigational drugs for the KEAP1 target, including 6 indications,8 R&D institutions involved, with related clinical trials reaching 1,and as many as 974 patents.
VVD-130037 is a drug being developed by Vividion Therapeutics, Inc. in the field of biomedicine. It targets KEAP1 and is indicated for the treatment of advanced malignant solid neoplasms. The drug has reached Phase 1 of clinical development, suggesting that it is currently being evaluated for its safety and tolerability in humans. Further research and clinical trials will be needed to determine the efficacy and potential therapeutic benefits of VVD-130037 in the treatment of advanced solid tumors.