Wave Life Sciences initiates Phase 2 FORWARD-53 trial, treating Duchenne Muscular Dystrophy patients with WVE-N531
Wave Life Sciences Ltd., an innovative enterprise operating in the clinical phase dedicated to developing transformative RNA-based therapies for individuals suffering from severe medical conditions, has officially commenced the administration of treatments in its Phase 2 FORWARD-53 study. This research investigates the efficacy of WVE-N531 as a therapeutic option for male patients with Duchenne muscular dystrophy, specifically those with genetic profiles suitable for exon 53 omission.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The FORWARD-53 study is aimed at evaluating the levels of functional dystrophin protein after treatment with biweekly doses of WVE-N531 at the 24-week and 48-week marks.
Anne-Marie Li-Kwai-Cheung, MChem, MTOPRA, RAPS, the Chief Development Officer at Wave Life Sciences, has expressed optimism following positive preliminary results from the initial exploratory studies with WVE-N531, noting that it could mark significant progress for Duchenne Muscular Dystrophy (DMD) by boosting the production of natural dystrophin protein to potentially alter the course of the condition.
”As the current FORWARD-53 trial incorporates biweekly administrations, the possibility of extending the dosing interval to once per month is a prospect for the future. We are buoyed by what WVE-N531 could bring to the table for DMD treatment,” added Li-Kwai-Cheung. She expressed gratitude for the DMD community's ongoing support and anticipation for the release of dystrophin expression outcomes in 2024, as well as the advancement of additional exon-skipping approaches for various mutations contingent upon FORWARD-53's success.
The FORWARD-53 trial, which is an open-label, Phase 2 clinical study potentially pivotal for registration purposes, includes 10 young male participants diagnosed with DMD who could benefit from exon 53 skipping treatments. The study has been set up to evaluate endogenous and functional dystrophin expression post-treatment at the 24-week and 48-week intervals, with biweekly intravenous administrations at a dose of 10 mg/kg. The trial's foremost outcome measurement is the level of dystrophin protein, and it's also examining pharmacokinetic aspects, digital and functional outcomes, along with safety and tolerance profiles.
Moreover, during the Research & Development Day hosted by Wave in September 2023, the company presented an analysis from muscle biopsy specimens collected during the initial Part A of the proof-of-concept study. This analysis suggested that WVE-N531 had integrated into myogenic stem cells, crucial for possible muscle restoration. Notably, these are the earliest clinical indicators, at six weeks after treatment, to show uptake into myogenic stem cells in DMD, further underpinning WVE-N531's potential to stand out from other treatment methodologies, including gene therapy options.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
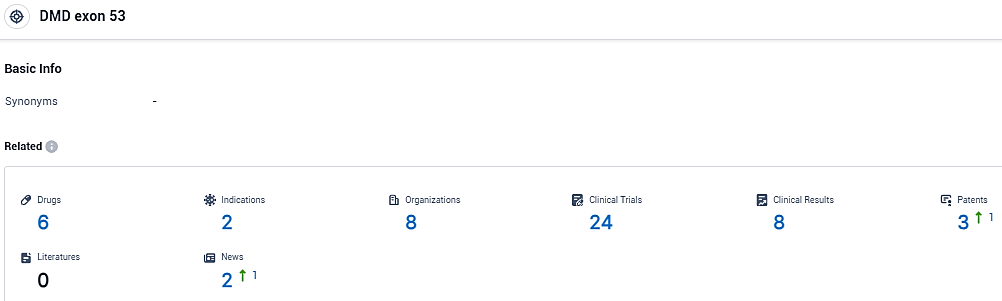
According to the data provided by the Synapse Database, As of December 27, 2023, there are 6 investigational drugs for the DMD exon 53 target, including 2 indications, 8 R&D institutions involved, with related clinical trials reaching 24, and as many as 3 patents.
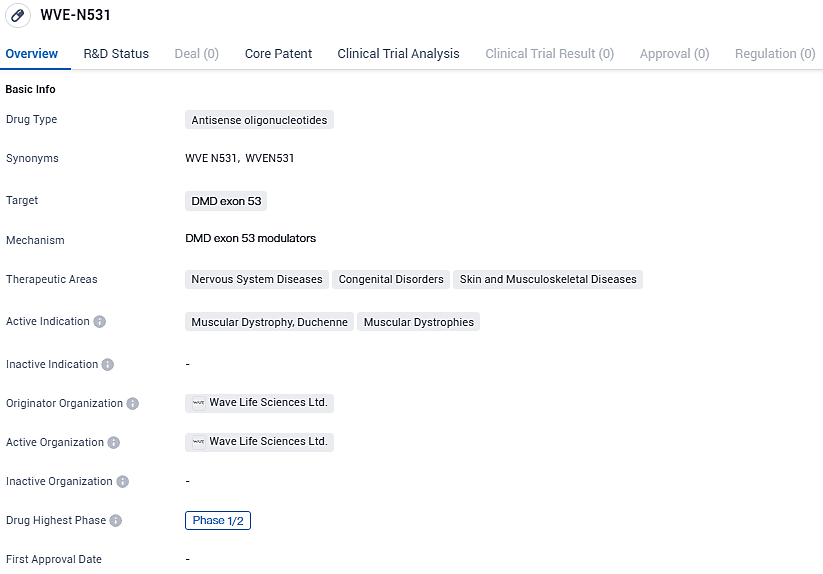
WVE-N531 targets DMD exon 53 and shows potential as a treatment for Muscular Dystrophy, Duchenne, and other Muscular Dystrophies. The drug is currently in Phase 1/2 of clinical development, and further research is needed to determine its efficacy and safety in larger patient population.