TQB2450: A Quick Look at Its R&D Progress and Clinical Results from the 2023 ESMO_ASIA
On 2 Dec 2023, the latest clinical trial about TQB2450 for the treatment for patients with hepatocellular carcinoma at high risk of recurrence was presented in 2023 ESMO_ASIA.
TQB2450's R&D Progress
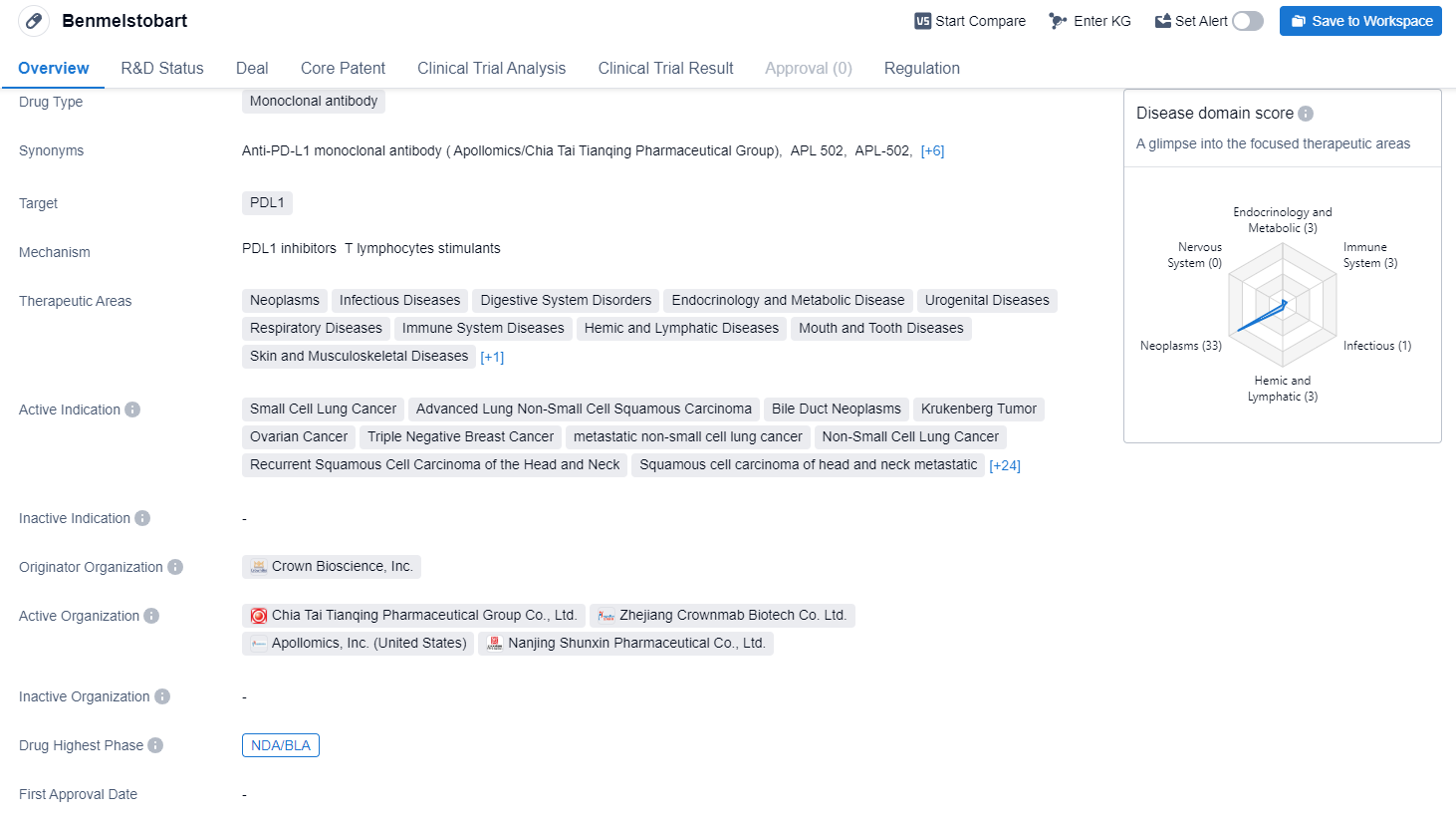
TQB2450, also called Benmelstobart, is a monoclonal antibody drug that targets PDL1. It has a wide range of therapeutic areas, including neoplasms, infectious diseases, digestive system disorders, endocrinology and metabolic disease, urogenital diseases, respiratory diseases, immune system diseases, hemic and lymphatic diseases, mouth and tooth diseases, skin and musculoskeletal diseases, and otorhinolaryngologic diseases.
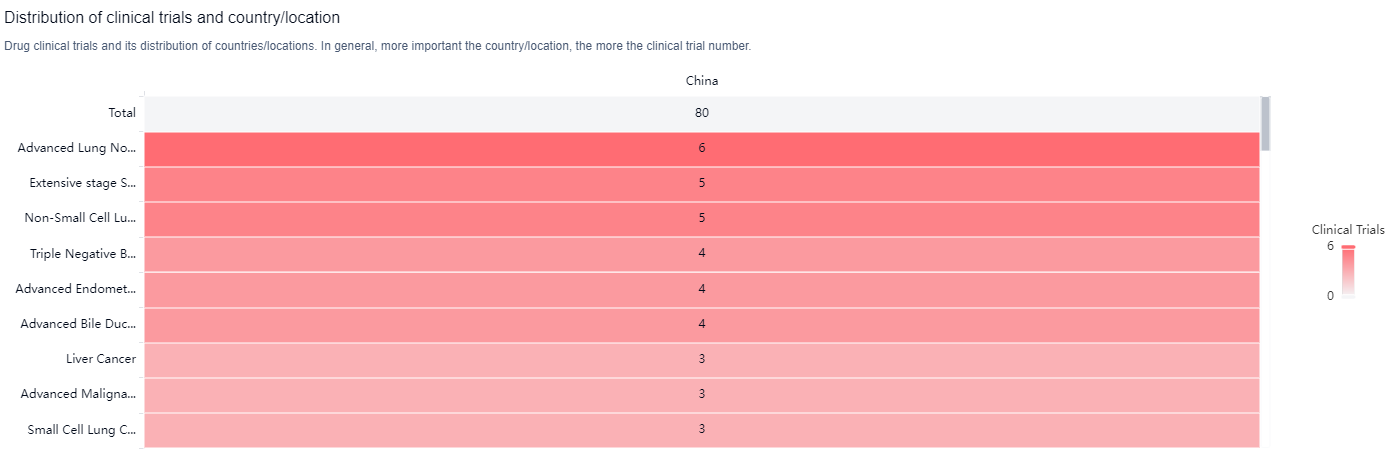
According to the Patsnap Synapse, TQB2450 has reached the highest phase of development, NDA/BLA globally. And the clinical trial distributions for TQB2450 are primarily in China. The key indication is Advanced Lung Non-Small Cell Carcinoma.
Detailed Clinical Result of TQB2450
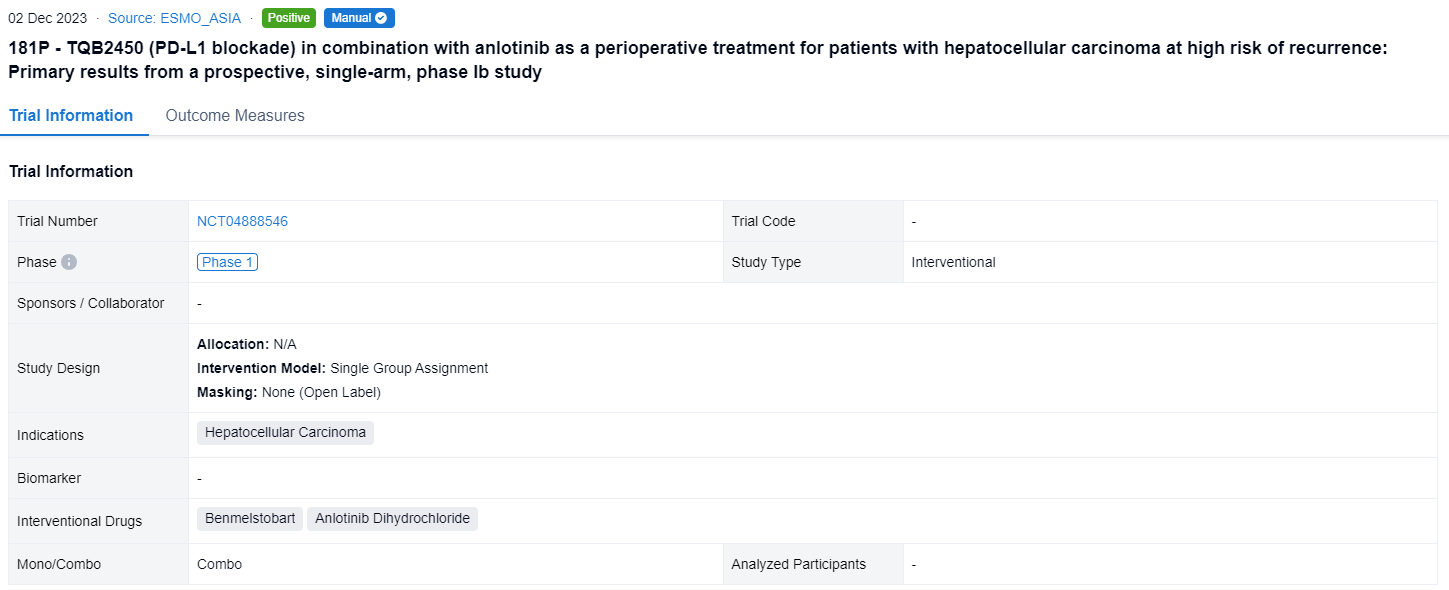
This single group assignment, open-labeled clinical trial (NCT04888546) was designed to evaluate the efficacy and safety of TQB2450 plus anlotinib as a perioperative regimen for the treatment of resectable HCC with a high risk of recurrence.
In this study, pts with primary resectable HCC who were at high risk of recurrence were enrolled. High-risk features include tumor size >5 cm, multiple tumors (≤ 3), satellite nodules, and macrovascular invasion (MVI). Before surgery, pts received 3 cycles of TQB2450 (1200 mg, IV, d1, Q3W) plus 2 cycles of anlotinib (10 mg, PO, d1-d14, Q3W). The feasibility of resection was assessed by radiographic imaging. After 30 days of surgery, patients continued combination therapy for 24 weeks. The primary endpoints were pCR and ORR (mRECIST). Secondary endpoints were PFS, OS, and safety. CRAFITY was derived from serum CRP and AFP values at baseline by adding one point each for CRP ≥1 mg/dL and AFP ≥100 ng/mL resulting in three categories: CRAFITY-low, 0 points; CRAFITY-intermediate, 1 point; CRAFITY-high, 2 points.
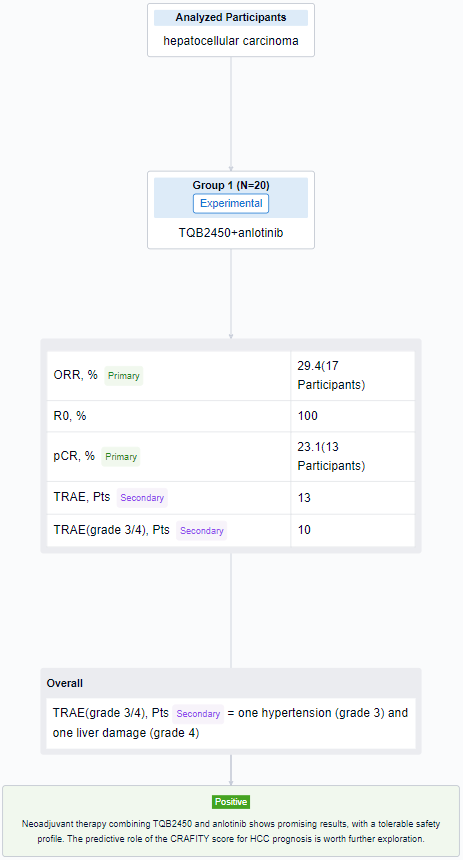
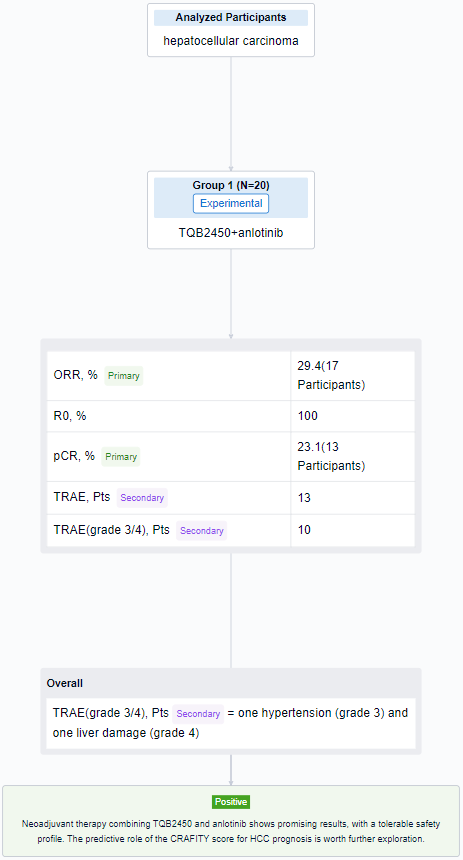
The result showed that as of July 15, 2023, 20 pts were enrolled (median age 61y [31-68], 95% male), 80% had HBV infection. All pts had Child-Pugh class A and ECOG PS 0. 11 (55%) had single (>5 cm) and 9 (45%) had multiple tumors. While n=10 (50%), n=8 (40%), and n=2 (10%) had CRAFITY-low, intermediate and high, respectively. Of 17 evaluable pts, ORR was 29.4% (5/17), 2 PR pts had low and 3 had intermediate CRAFITY scores. 13 pts completed preoperative therapy and underwent hepatic resection, the R0 resection rate was 100% and the pCR rate was 23.1% (3/13). Among the 3 pCR pts, 1 had low and 2 had intermediate CRAFITY scores; 2 had multiple tumors and no pts had MVI. 13 pts had TRAEs and grade 3/4 TRAEs (10%) including one hypertension (grade 3) and one liver damage (grade 4).
It can be concluded that neoadjuvant therapy combining TQB2450 and anlotinib shows promising results, with a tolerable safety profile. The predictive role of the CRAFITY score for HCC prognosis is worth further exploration.
How to Easily View the Clinical Results Using Synapse Database?
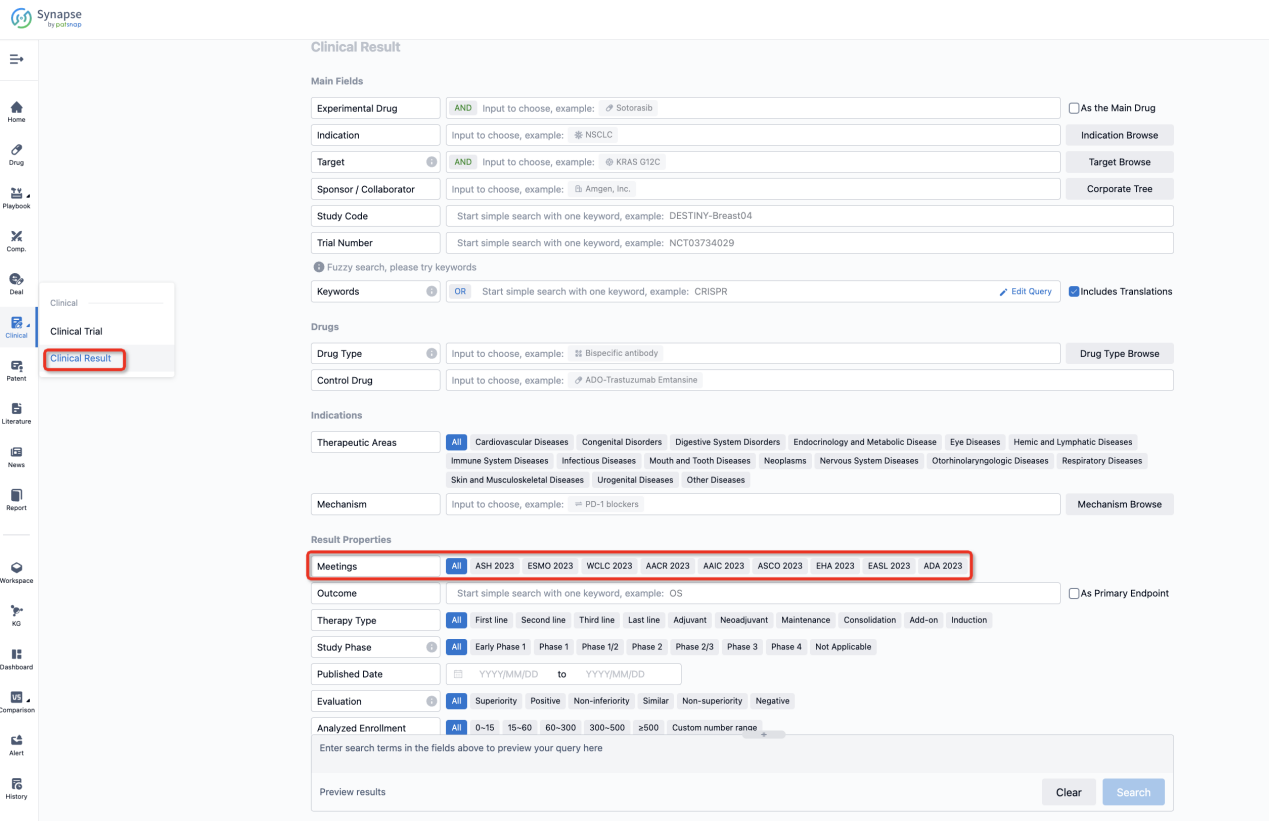
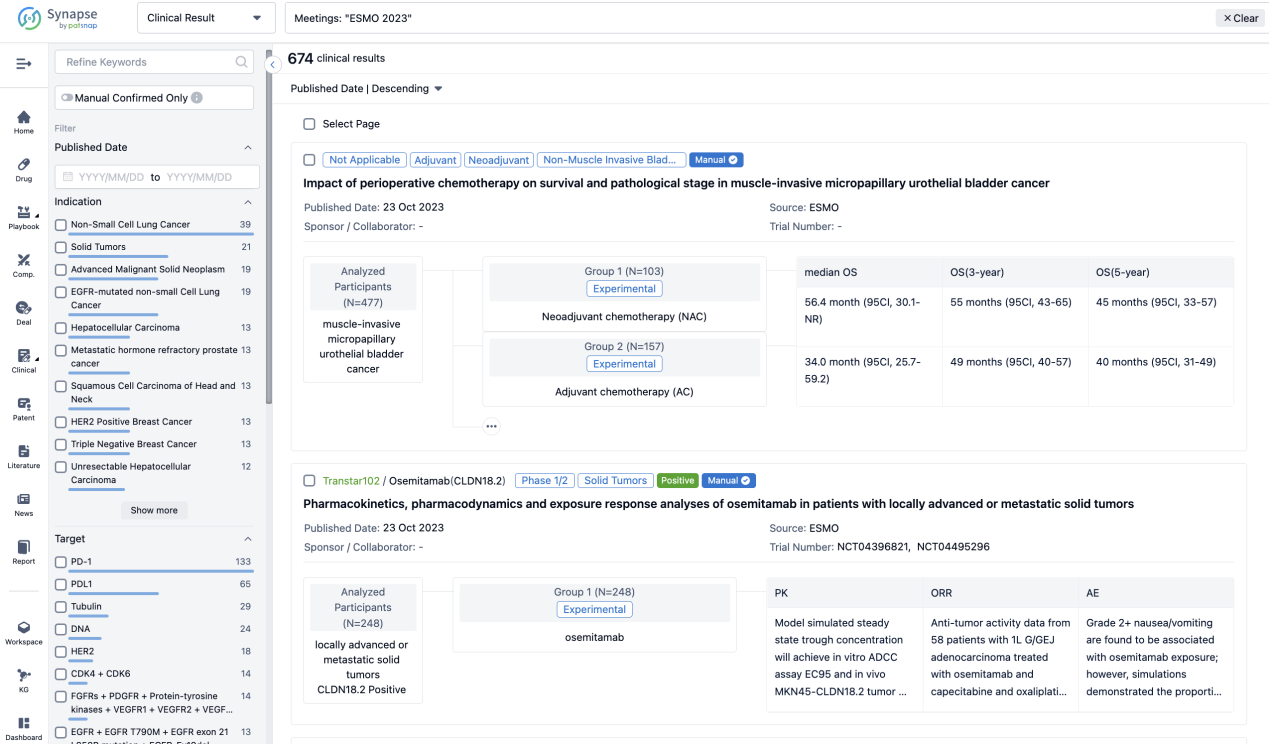
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
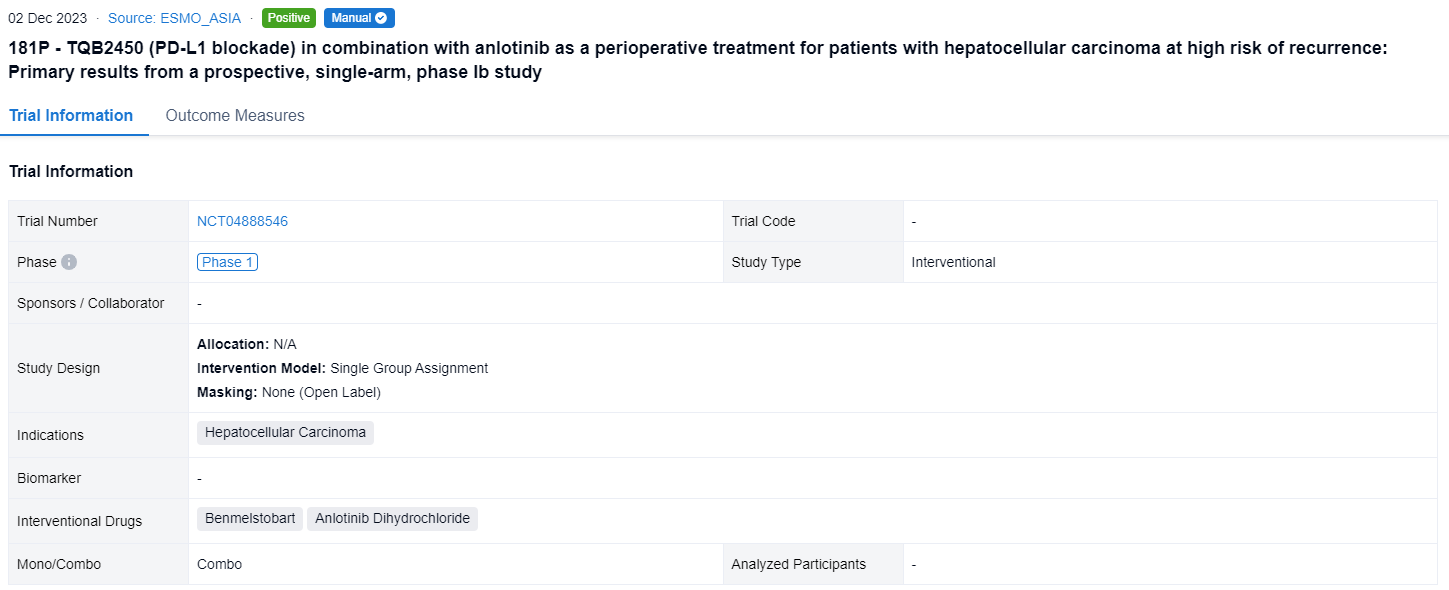
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
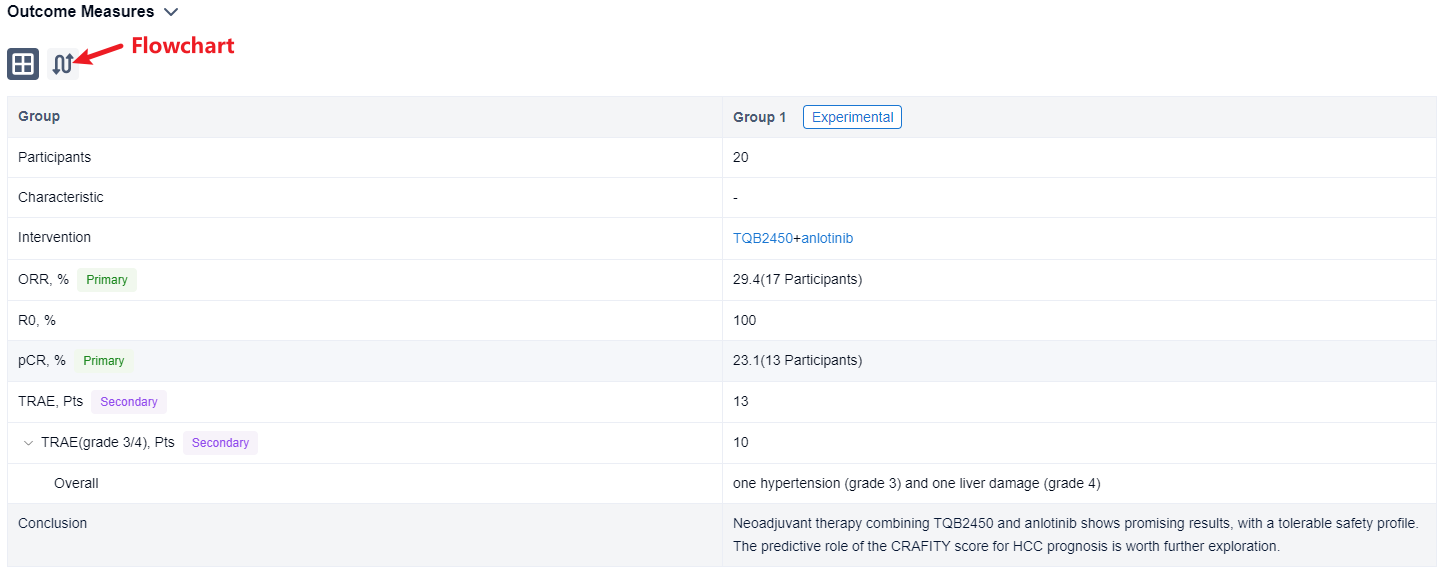
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
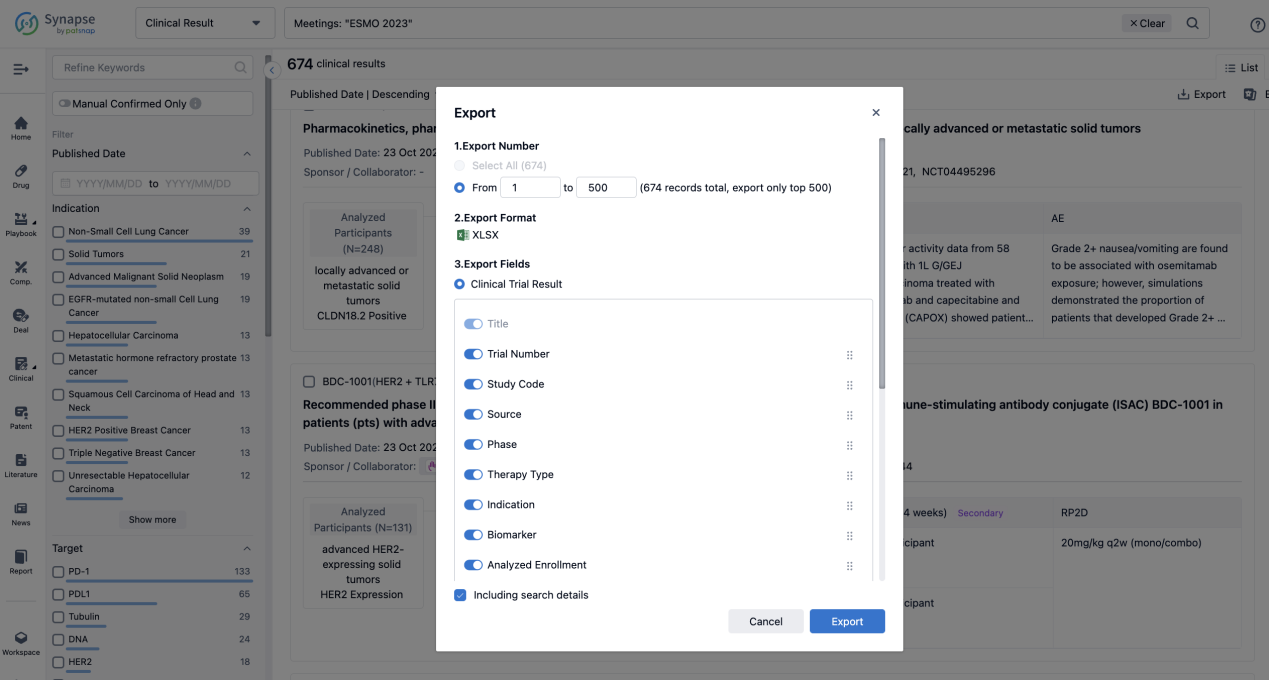
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!