What ADC-related progress will Keymed Biosciences reveal at the ASCO conference?
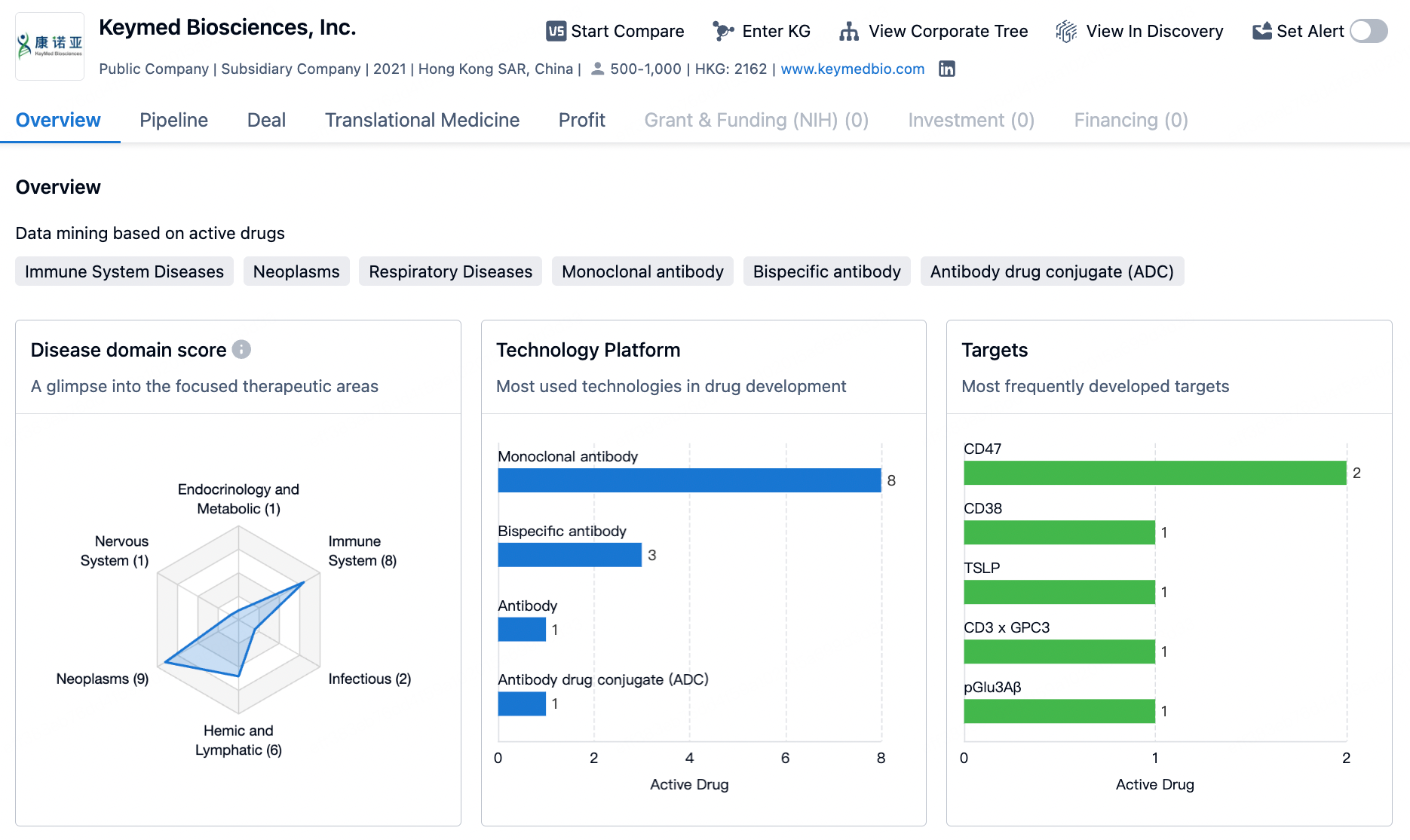
Keymed Biosciences is a biotech company focused on the innovative research and development of autoimmune and oncology therapeutics. The company has established four core technology platforms, including: the novel T Cell Engager (nTCE) platform, a bioevaluation platform, an innovative antibody discovery platform, and a high-throughput screening platform for high-yield antibody drug expression cells. The company's pipeline primarily covers the fields of autoimmunity and oncology, with over 30 class I innovative drugs under development, 9 of which have entered various clinical research phases.
CMG901 (AZD0901) is a potentially first-in-class antibody-drug conjugate targeting Claudin 18.2, conjugated with monomethyl auristatin E (MMAE) as the payload. It is currently undergoing Phase I clinical studies in patients with advanced solid tumors, including gastric and pancreatic cancers. Recently, Keymed Biosciences announced that AstraZeneca has initiated multiple clinical studies for the treatment of advanced solid tumors with CMG901; an international multi-center Phase III study comparing AZD0901 alone versus investigator-chosen regimens as second or later line treatment in participants with advanced or metastatic gastric and gastroesophageal junction adenocarcinoma expressing Claudin 18.2 was registered on the Clinical Trial Registration and Information Platform in March 2024, with the first participant receiving their initial dose on April 11, 2024.
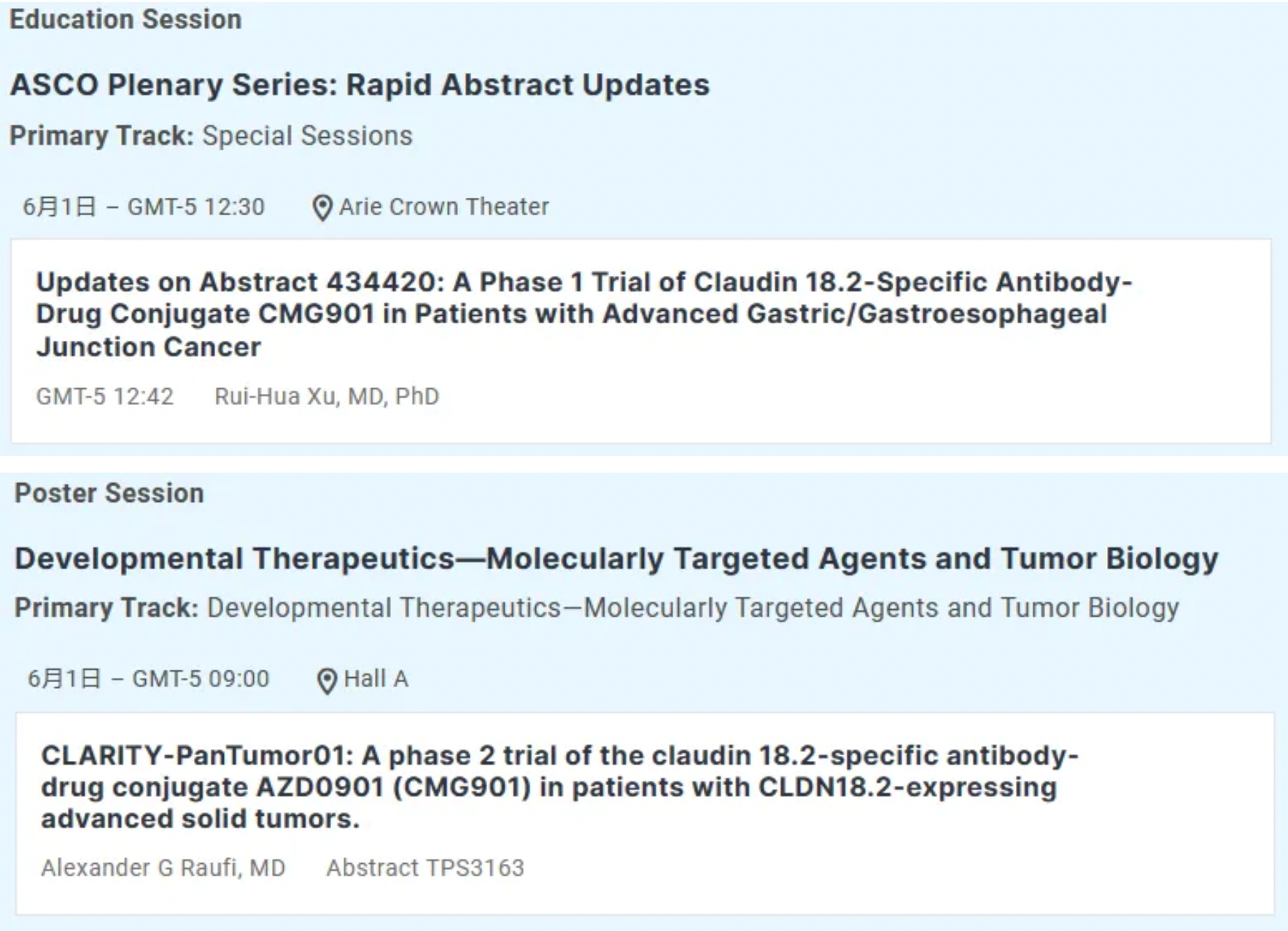
Previously, AstraZeneca and Keymed Biosciences entered into an exclusive worldwide licensing agreement for the potentially first-to-market antibody-drug conjugate targeting Claudin 18.2, CMG901. Subsidiary KYM Biosciences will receive a $63 million upfront payment along with potential additional R&D and sales-related milestone payments exceeding $1.1 billion, as well as tiered royalties in the low double digits.
In November 2023, Keymed Biosciences reported the latest data from the Phase I clinical study of CMG901 in advanced gastric or gastroesophageal junction adenocarcinoma. As of July 24, 2023, 113 patients were enrolled across three dosing groups - 2.2mg/kg, 2.6mg/kg, and 3.0mg/kg (comprising 44, 50, and 19 cases respectively). All participants had received at least one line of standard treatment previously (with a median prior line of treatment being second-line), and 74% had previously undergone anti-PD-1/PD-L1 therapy. The results showed that among 89 evaluable Claudin 18.2 positive gastric or gastroesophageal junction adenocarcinoma patients, the confirmed objective response rate (ORR) was 33%, and the disease control rate (DCR) was 70% across all three dosage groups. In the 2.2mg/kg dose group, the confirmed ORR was 42%, the median progression-free survival (mPFS) was 4.8 months, and the median overall survival (mOS) had not yet been reached. Regarding safety, the incidence of ≥ grade 3 treatment-related adverse events was 54%, the rate of serious treatment-related adverse events was 31%, and 8% of participants discontinued due to drug-related adverse events.
How to search for and analyze the development progress of ADC pharmaceuticals?
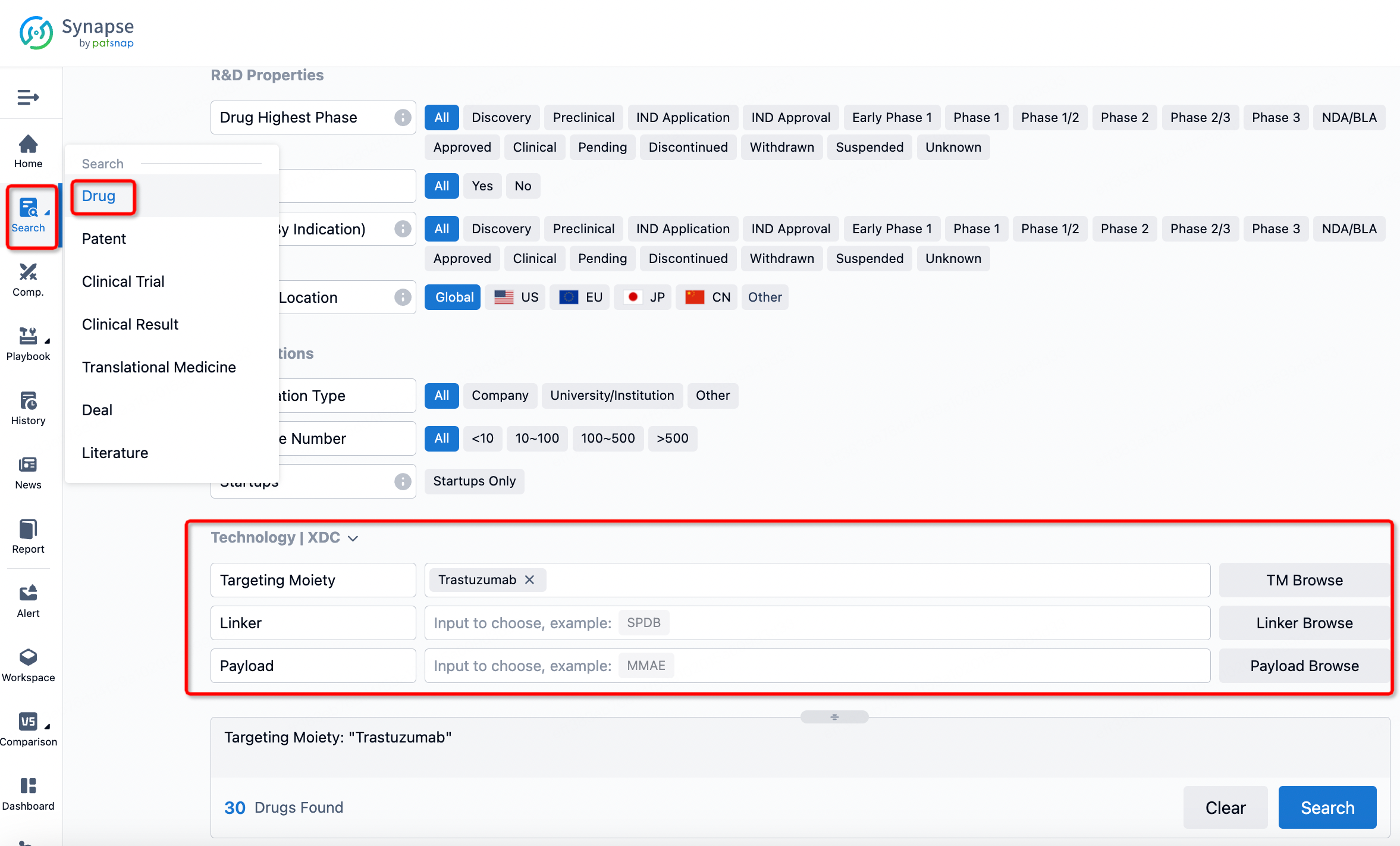
If you want to learn about the latest developments in ADC drugs, you can use the drug search module of the Synapse database. This module supports searching for ADC drugs by classification through Targeting Moiety, Linker, and Payload.
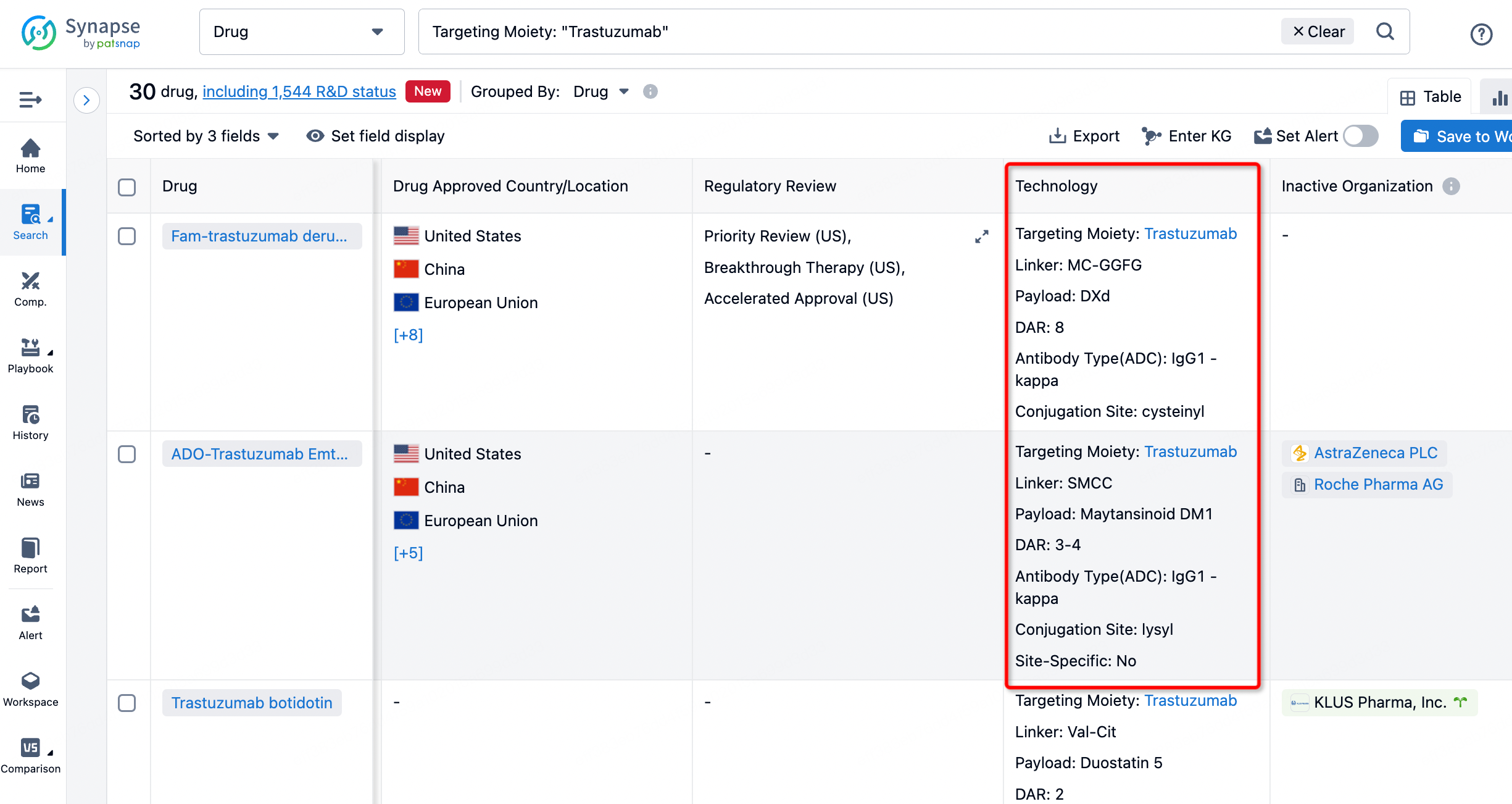
On the search results page, you can easily review information related to the ADC's technical category of the drugs.
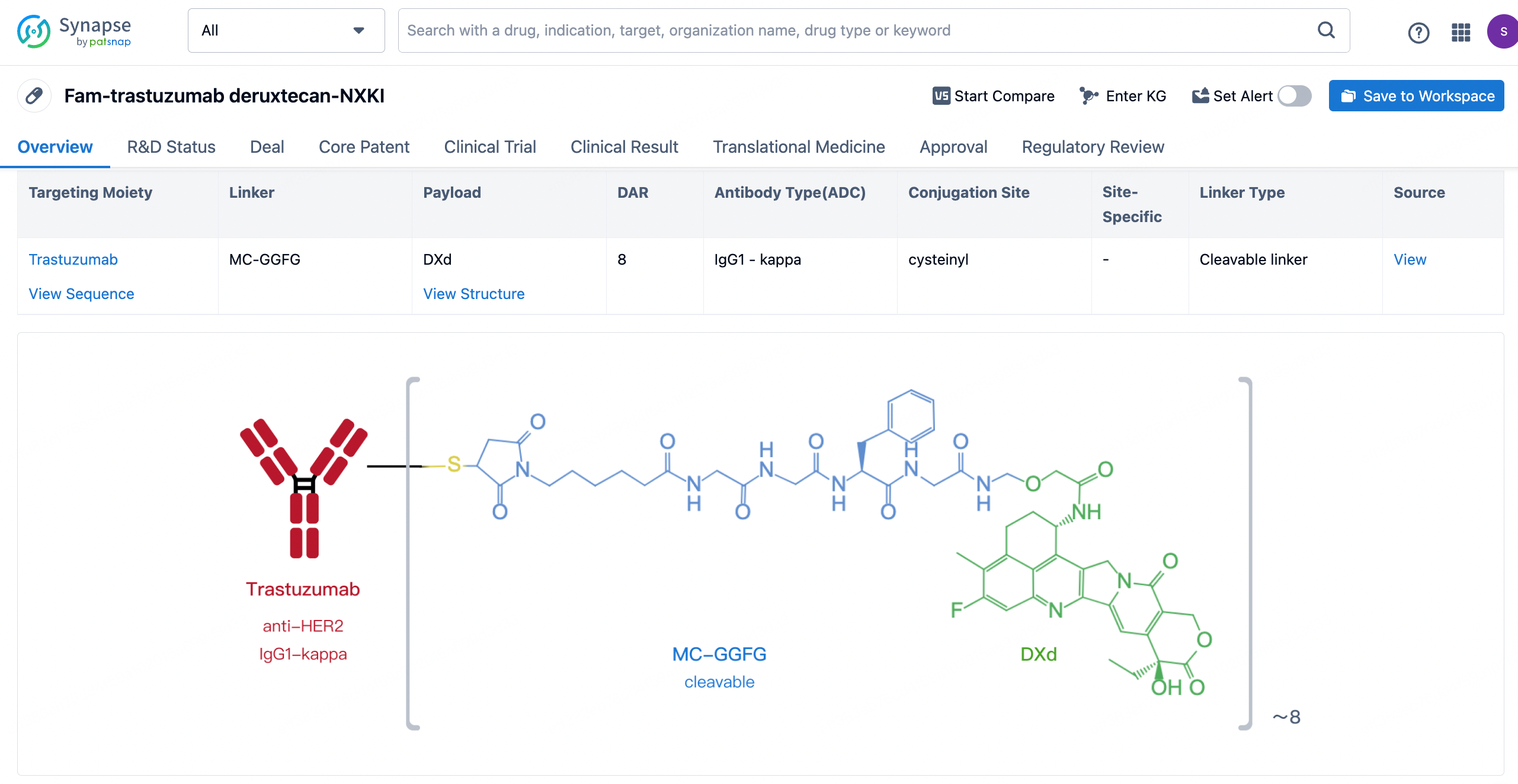
After clicking to enter the drug details page, you can also effortlessly obtain structural information about the ADC drug.
Click on the image below to embark on a brand new journey of drug discovery!