What Retrieval Methods Can be Used in the CAR-T Field to Find More Comprehensive Technical Materials and Patent Information? (Part II)
In the CAR-T field, we can perform comprehensive data retrieval of CAR-T in two major parts: antibody search and CAR expression cassette design.
Antibody Search: To enable CAR-T to reach its target successfully, choosing the appropriate antigen recognition domain is essential.
CAR Expression Cassette: The design of the CAR expression cassette determines whether a T cell can become CAR-T and the effect it produces.
The previous content thoroughly explained the retrieval strategy for the antibody searching section: What Retrieval Methods Can Be Used in the CAR-T Field to Find More Comprehensive Technical Materials and Patent Information? (Part 1). In this content, we mainly discuss the search for the CAR expression cassette. Data retrieval can be done primarily through the following aspects:
Sequences Search——based on the drug sequence, similar sequences are retrieved, also searching related patents and literature information.
Fragment retrieval——supports searching for related sequences based on core components of CAR-T.
Combined searching——searching for patents containing multiple sequences at once.
Let’s look at the specific retrieval methods:
I. Cases where the core functional elements of CAR in the patent are written in one sequence.
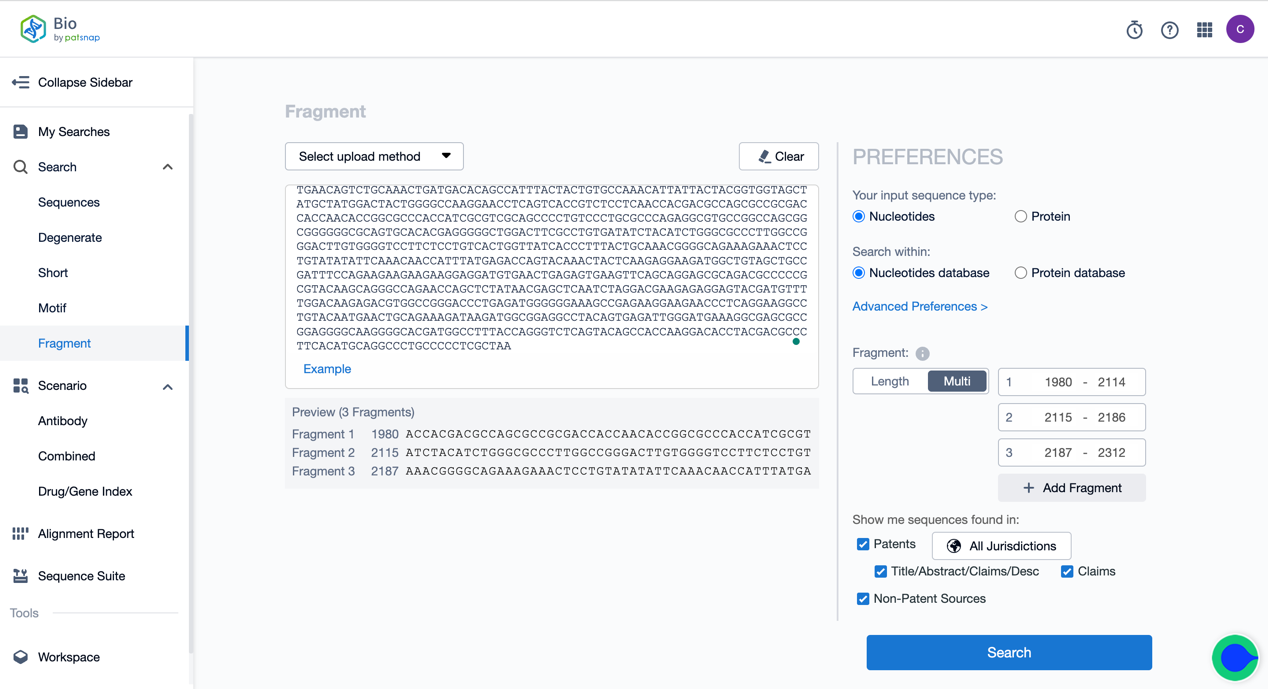
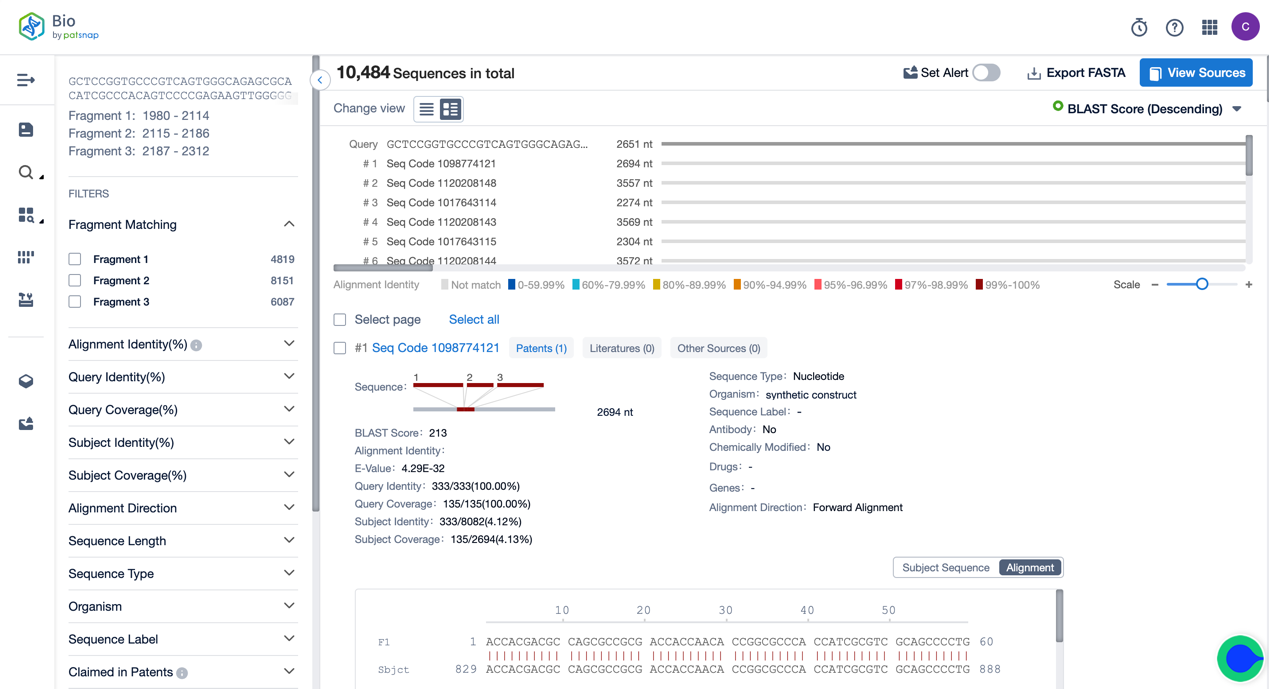
One can take advantage of the fragment search function of the Bio database for retrieval. Firstly, register a Patsnap Bio Sequence Database account for free. Then, enter the whole sequence of Tisagenlecleucel into the fragment search function on the database homepage, and choose the multi-segment fragment (currently supporting a maximum selection of 10 segments) in the options on the right. We assign the starting and termination points of the Hing, Transmembrane, and Intracellular sections, respectively. Click search, and you can look up the related sequence results of the three core functional components of Tisagenlecleucel.
II. Cases where the core functional elements of CAR in the patents are written separately.
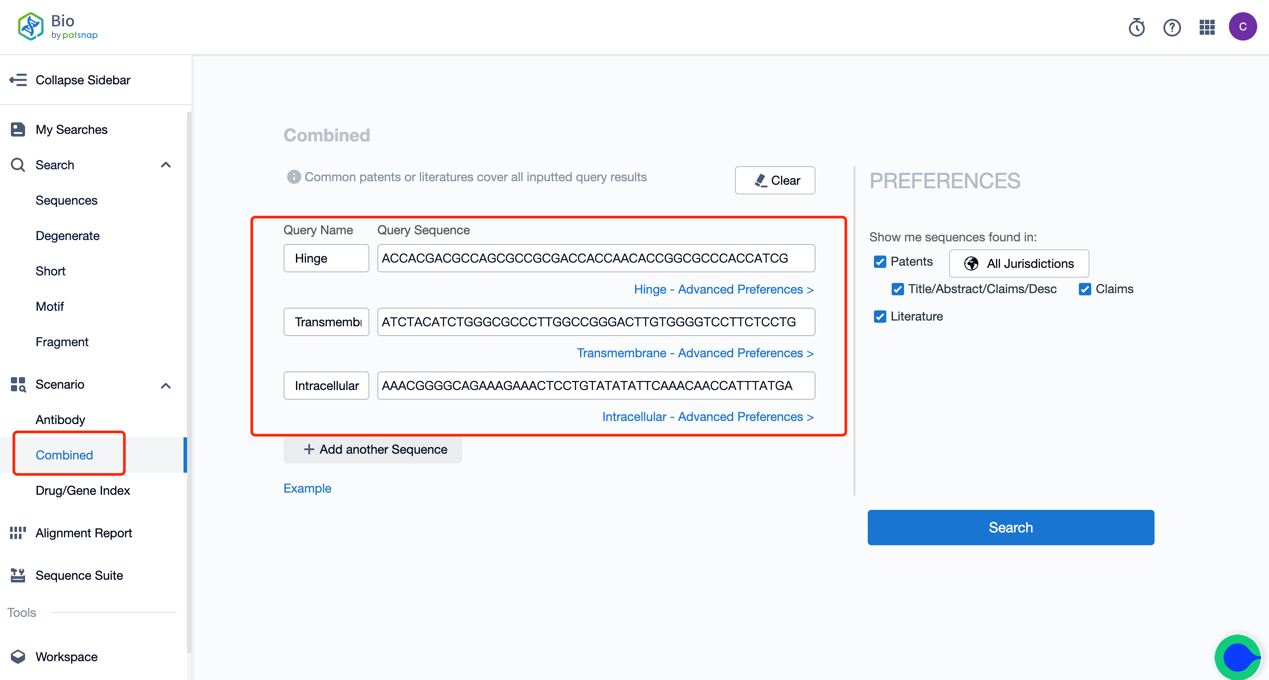
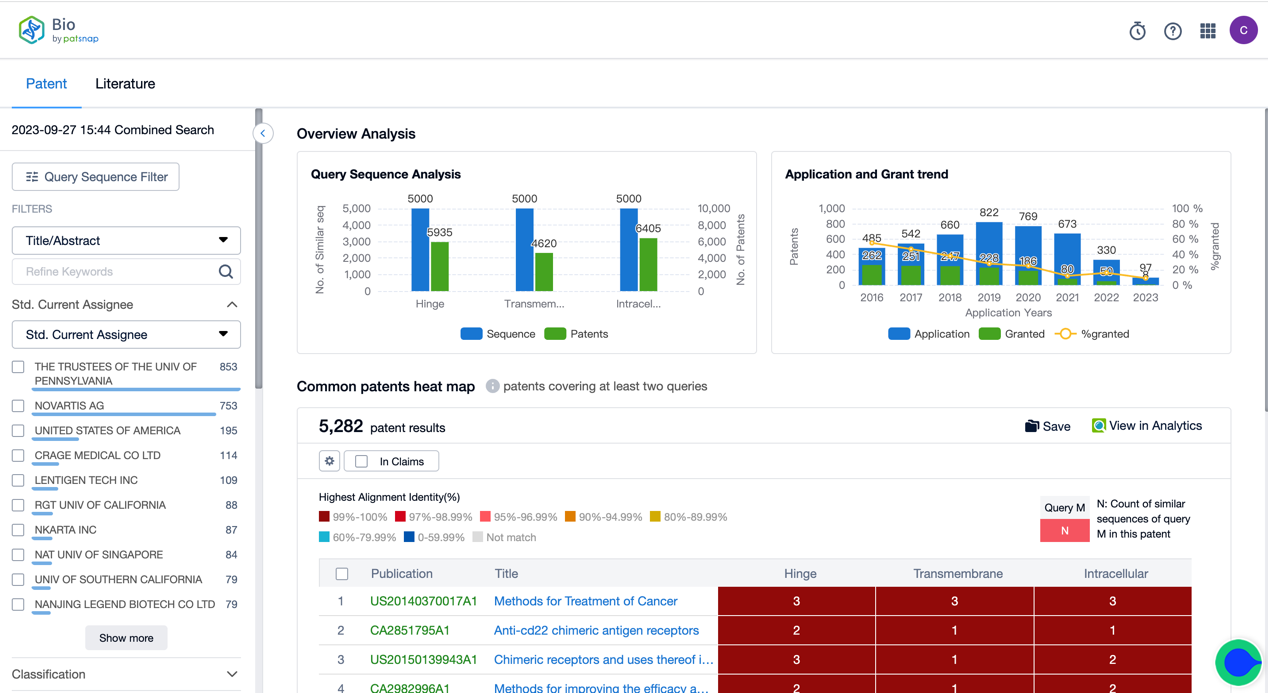
The combined search function of the Bio database can be utilized for retrieval, searching for patents that simultaneously release combinations of multiple sequences based on the CAR-T core functional elements in the patents, such as the antigen-recognizing area, hinge region, transmembrane region, and intracellular structure domain, etc. We input the three parts of Tisagenlecleucel’s Hing, Transmembrane, and Intracellular separately into the search box (supporting a maximum of 6 sequence combinations for now) and then click search to view the heat map of patents that release the three sequences at once and then further enter the patent to view the details. In the combined search's result filtering options, we can customize 1-3 sequence combinations to view the results you want precisely.
The aforementioned are all ways to retrieve sequences of patents, literature, and other technical data in the CAR-T field.
Regardless of whether you are personnel conducting patentability analysis and FTO work for CAR-T output sequences or personnel who are engaged in CAR-T drug development research and development, you can consult and verify the respective patent technical information accurately and comprehensively in the Patsnap Bio sequence database utilizing various retrieval functions and methods.
It is important to note that Patsnap Bio is the most extensive sequence search platform for the Patsnap database. It incorporates AI with human-curated data for comprehensive handling of protein and nucleotide sequence data plucked from global patents, biological periodicals, and public repositories. Essential biological sequences are manually annotated, illuminating structural modifications to provide the most accurate sequence data and boost sequence retrieval efficiency.
Free registration is available for the Bio biological sequence database: https://bio.patsnap.com. Act now to expedite your sequence search tasks.