Yescarta® Car T-cell therapy from Kite greatly boosts treatment response and prolongs remission in relapsed/resistant Large B-cell Lymphoma patients
Kite, a subsidiary of Gilead Company, revealed the findings of the ALYCANTE Phase 2 study. The research, orchestrated and financed by the French cooperative group LYSA/LYSARC, regards the application of their chimeric antigen receptor T-cell therapy Yescarta®(axicabtagene ciloleucel) for individuals with relapsed/refractory large B-cell lymphoma. These patients had previously undergone a single line of therapy but were considered unsuitable for intensive chemotherapy and autologous stem cell transplantation. Nature Medicine published the complete results of this study.
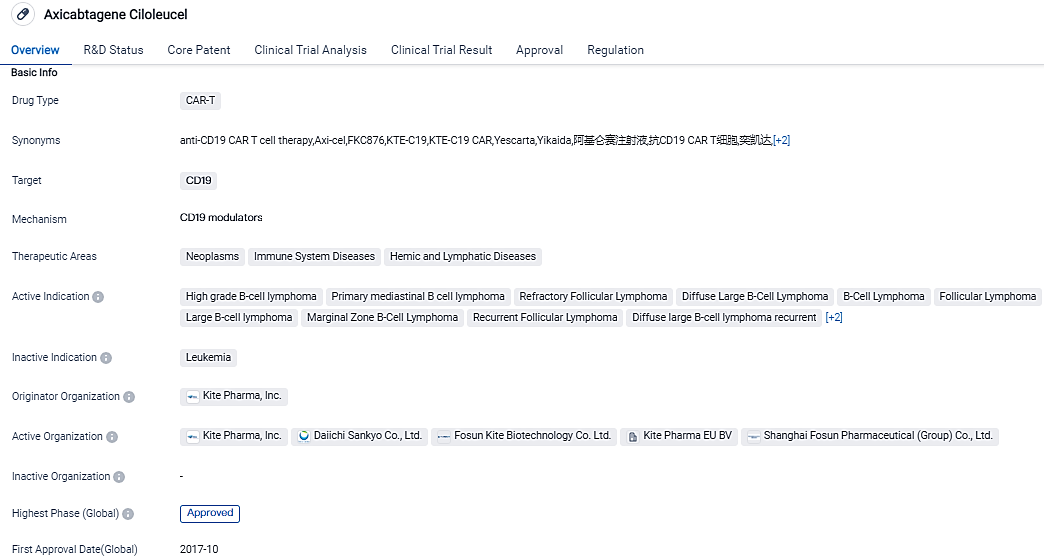
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In the ALYCANTE research, a Phase 2 trial conducted by LYSA across multiple centers, Yescarta's effectiveness and safety as a secondary treatment method were assessed for the first time on 62 R/R LBCL patients who were unfit for HDCT and ASCT. The primary objective of the study was achieved, showing a full metabolic response of 71% at three months compared to the anticipated 12% with the typical care standard. At the six-month mark, 59.7% of patient's upheld CMR. CMR is described as having no detections in a PET scan during or post-antitumor treatment.
Professor Roch Houot, leader of the Hematology Department at Univserity Hopspital of Rennes, France, and ALYCANTE research coordinator commented, "ALYCANTE is the maiden study observing axicabtagene ciloleucel as a secondary line of treatment for transplant unfit R/R LBCL patients who face dire prognosis due to their aggressive relapsed or refractory B-cell lymphomas. The outcomes have unveiled elevated response rates and sustained remission in this difficult group”.
Dr. Frank Neumann, MD, PhD, SVP, and Clinical Development Global Head at Kite, concluded, "For those patients who are classed as unfit for stem cell surgery, the findings from ALYCANTE confirm the consideration of Yescarta as another treatment solution, potentially curing the condition. The continuous research and findings for Yescarta uphold its potential to offer hope to patients facing various forms of large B-cell lymphoma and follicular lymphoma."
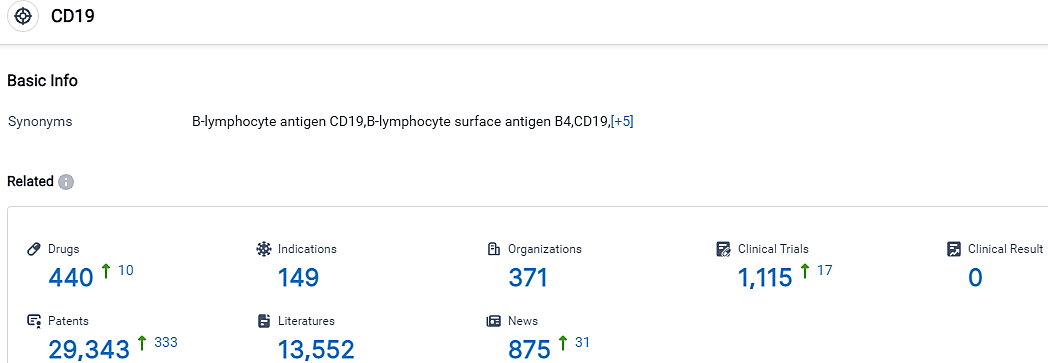
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 20, 2023, there are 440 investigational drugs for the CD19 target, including 149 indications,371 R&D institutions involved, with related clinical trials reaching 1115,and as many as 29343 patents.
Axicabtagene ciloleucel is a CAR-T therapy drug, it targets CD19 and is used in the treatment of various neoplasms, immune system diseases, and hemic and lymphatic diseases. The drug has received approvals in the United States and China and has reached the highest phase of development. It has been granted priority review, breakthrough therapy designation, and orphan drug status.