Zai Lab will showcase research on its innovative ADC therapy, ZL-1310, for combating solid cancers
Zai Lab Limited has declared its intention to showcase results from early-stage experimental research that emphasize the possible therapeutic benefits of ZL-1310, the organization's worldwide advanced antibody-drug conjugate initiative. During the upcoming European Lung Cancer Congress 2024, taking place between March 20th and 23rd, 2024 in Prague, Czech Republic, an informative poster session will delve into the preclinical characteristics of ZL-1310.
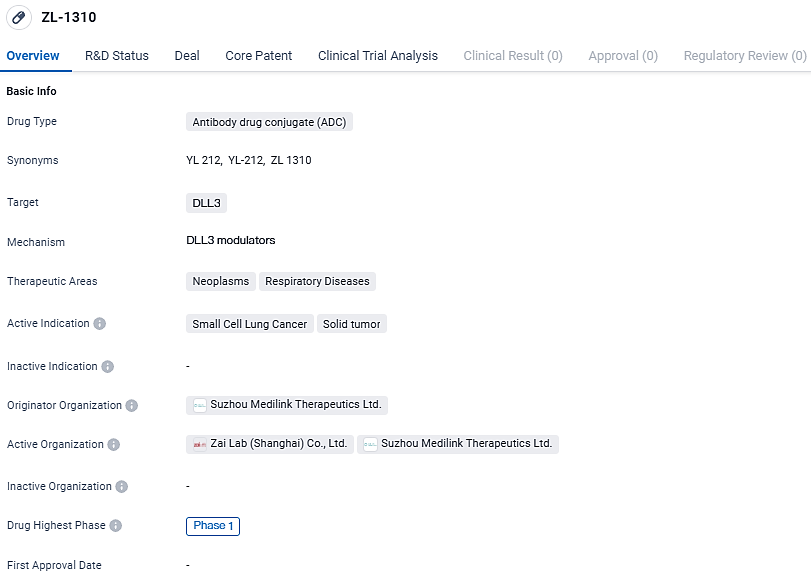
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
ZL-1310 emerges as a cutting-edge antibody-drug conjugate (ADC) being developed by Zai Lab, aimed at Delta-like ligand 3 (DLL3) – a known treatment target for small cell lung cancer (SCLC). DLL3 plays an integral role in SCLC due to its presence on the majority of SCLC cell surfaces and its involvement in the increase of SCLC cell growth, mobility, and invasive capabilities.
"ZL-1310 demonstrates a pronounced ability to recognize and bind to DLL3, initiating the release of its toxin both inside cancer cells and within the tumor milieu, thus facilitating a collateral destruction of neighboring malignant cells via its camptothecin-based cytotoxin," noted Rafael G. Amado, M.D., who leads Zai Lab’s Global Oncology Research and Development as president. Dr. Amado also mentioned that compelling preclinical results support the foundational study of ZL-1310, which propelled the commencement of its Phase 1 trial as of January this year.
Anticipated to be highlighted at ELCC 2024, early-stage non-clinical studies have shown that ZL-1310 latches onto DLL3 with high specificity, instigating its ingestion by SCLC cells, stalling their proliferative cycle, and triggering programmed cell death. In living organisms, ZL-1310 has proven efficacy in diminishing the growth of implanted human tumors in models derived from both SCLC cell lines and patient samples, with effects intensifying with higher dosages.
The conception of ZL-1310 was facilitated through MediLink Therapeutics’ TMALIN® technology, an advanced ADC platform that strategically harnesses the tumor environment. This innovative platform is meant to resolve some of the pressing challenges faced by existing ADCs. Zai Lab secured an exclusive partnership and worldwide licensing deal with MediLink in April 2023. This collaboration paved the way for the international Phase 1 clinical investigation of ZL-1310, specifically in SCLC patients who showed disease progression despite receiving platinum-based chemotherapies, which Zai Lab initiated in January 2024.
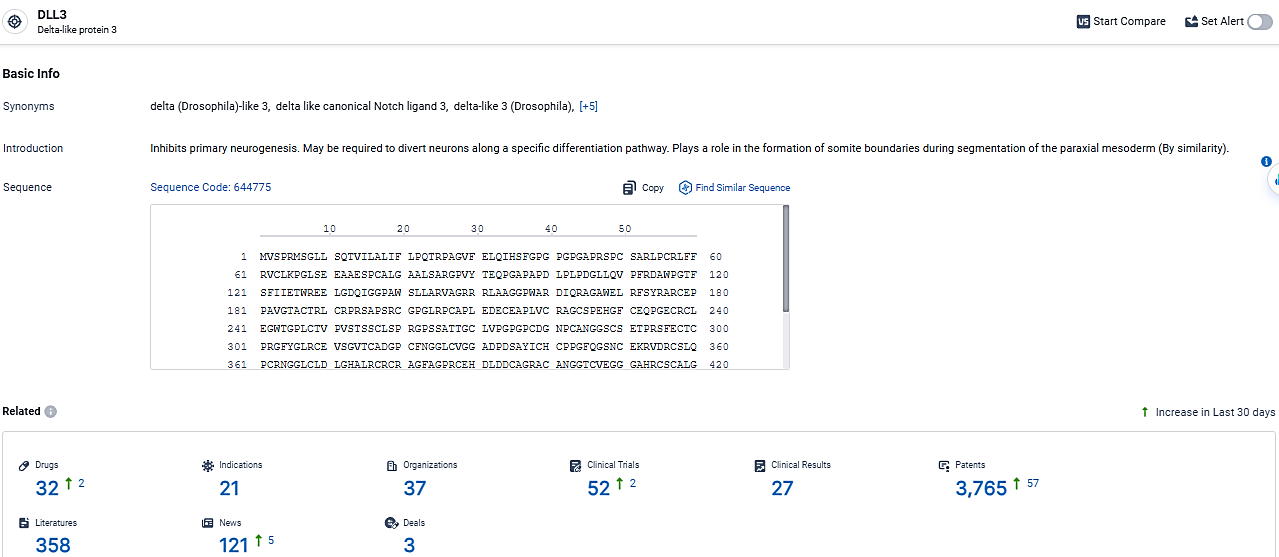
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 17, 2024, there are 32 investigational drugs for the DLL3 target, including 21 indications, 37 R&D institutions involved, with related clinical trials reaching 52, and as many as 3765 patents.
ZL-1310 is currently in Phase 1 of clinical trials, both globally and in China, with a focus on treating neoplasms and respiratory diseases, specifically small cell lung cancer and solid tumors. Further research and development will be needed to determine the efficacy and safety of ZL-1310 in larger patient populations.