Zealand Pharma Announces Positive Results from 13-Week Phase 1b Trial of Dapiglutide
Zealand Pharma A/S, a biotechnology firm specializing in the discovery and development of novel peptide-based therapies, has reported encouraging topline outcomes from a Phase 1b multiple ascending dose (MAD) study. This trial was designed to assess the safety, tolerability, and clinical impact of 13 weeks of dapiglutide administration, a long-acting GLP-1/GLP-2 receptor dual agonist currently being developed for weight management purposes.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 "We are both enthusiastic and highly optimistic about the data from this short-term trial, which demonstrates significant and clinically meaningful reductions in body weight," stated David Kendall, MD, Chief Medical Officer of Zealand Pharma. "Preliminary findings further suggest that dapiglutide offers a distinctive profile due to its unique dual agonist properties, potentially leading to beneficial effects on inflammation. We are now investigating the potential for higher doses of dapiglutide, up to 26 mg, over an extended treatment period of 28 weeks in Part 2 of this ongoing trial, with topline results expected in the first half of 2025. Importantly, these promising data provide the confidence to rapidly advance dapiglutide into a comprehensive Phase 2b trial targeting individuals with overweight and obesity, slated to commence in the first half of 2025."
"We are both enthusiastic and highly optimistic about the data from this short-term trial, which demonstrates significant and clinically meaningful reductions in body weight," stated David Kendall, MD, Chief Medical Officer of Zealand Pharma. "Preliminary findings further suggest that dapiglutide offers a distinctive profile due to its unique dual agonist properties, potentially leading to beneficial effects on inflammation. We are now investigating the potential for higher doses of dapiglutide, up to 26 mg, over an extended treatment period of 28 weeks in Part 2 of this ongoing trial, with topline results expected in the first half of 2025. Importantly, these promising data provide the confidence to rapidly advance dapiglutide into a comprehensive Phase 2b trial targeting individuals with overweight and obesity, slated to commence in the first half of 2025."
In Part 1 of the Phase 1b trial, 54 participants (approximately 85% male) with a median age of 46 years and a median baseline BMI of 30.0 kg/m² were randomly assigned to receive either dapiglutide or placebo (14:4) across three dose groups for 13 weeks. By week 13, the average body weight had decreased by up to 8.3% on a placebo-corrected basis among those treated with dapiglutide (with a mean weight loss of up to 6.2% on dapiglutide and a mean weight gain of 2.1% on placebo). No lifestyle interventions, such as dietary changes or physical activity, were included in the trial.
Dapiglutide was found to be safe and well-tolerated in Part 1, with no severe treatment-emergent adverse events (TEAEs) and a single serious adverse event that was not associated with the drug. The most frequent TEAEs involved gastrointestinal issues, such as nausea and vomiting. Only two participants discontinued the treatment due to gastrointestinal-related AEs (moderate vomiting). Overall, the gastrointestinal events observed were in line with those seen in other incretin-based therapy clinical trials with a similar, rapid dose escalation protocol. Injection site reactions were reported by a small number of participants, all of which were mild. Anti-drug antibodies were detected in 14.3% of participants.
Part 2 of this Phase 1b trial assesses higher doses of dapiglutide, up to 26 mg, over a 28-week treatment duration with dose increases every fourth week. Zealand anticipates presenting topline results from Part 2 in the first half of 2025 and plans to share additional data from both Part 1 and Part 2 at an upcoming scientific conference. The company intends to continue the clinical development of dapiglutide into a Phase 2b trial for individuals with overweight and obesity, with the trial start expected in the first half of 2025. Additionally, the company aims to explore the potential of dapiglutide in specific obesity-related comorbidities.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
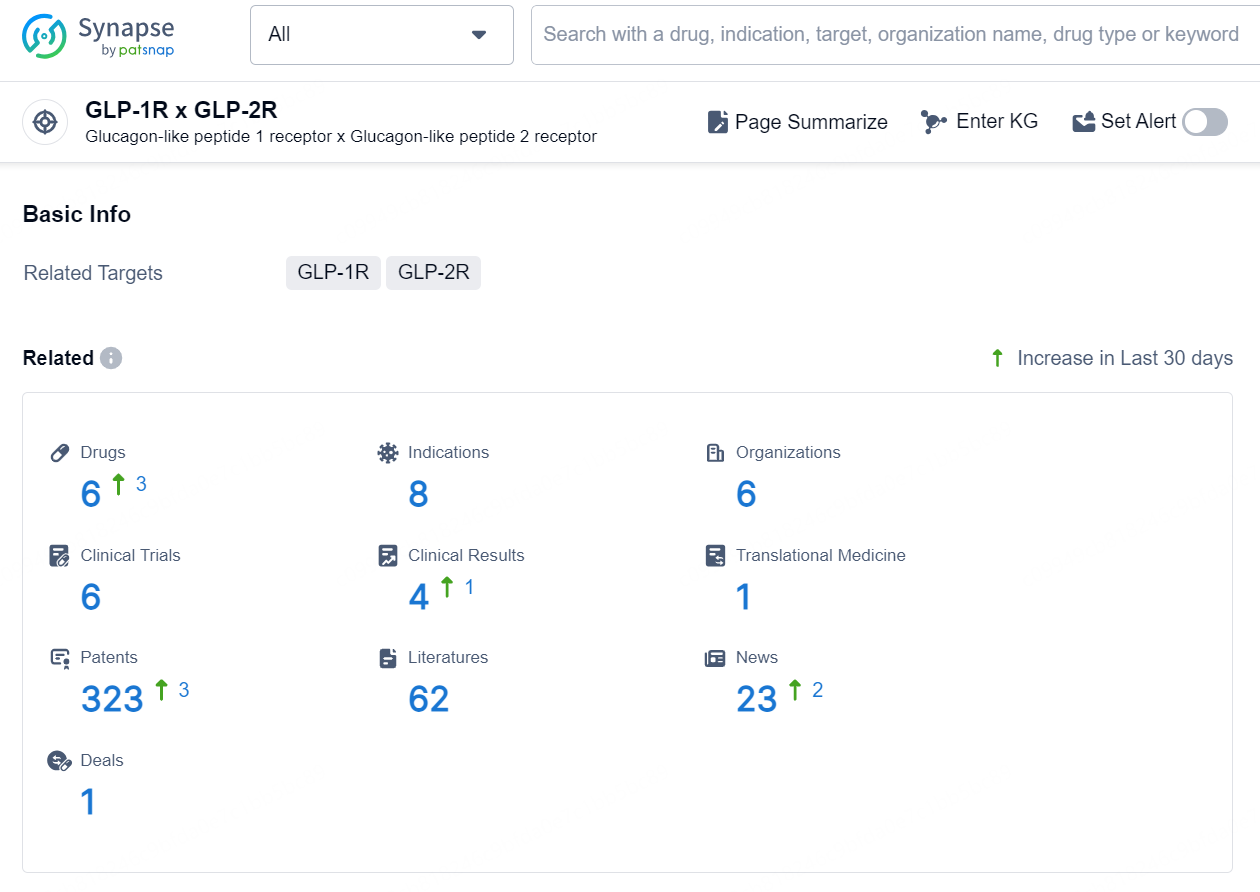 According to the data provided by the Synapse Database, As of September 14, 2024, there are 6 investigational drugs for the GLP-1R x GLP-2R target, including 8 indications, 6 R&D institutions involved, with related clinical trials reaching 6, and as many as 323 patents.
According to the data provided by the Synapse Database, As of September 14, 2024, there are 6 investigational drugs for the GLP-1R x GLP-2R target, including 8 indications, 6 R&D institutions involved, with related clinical trials reaching 6, and as many as 323 patents.
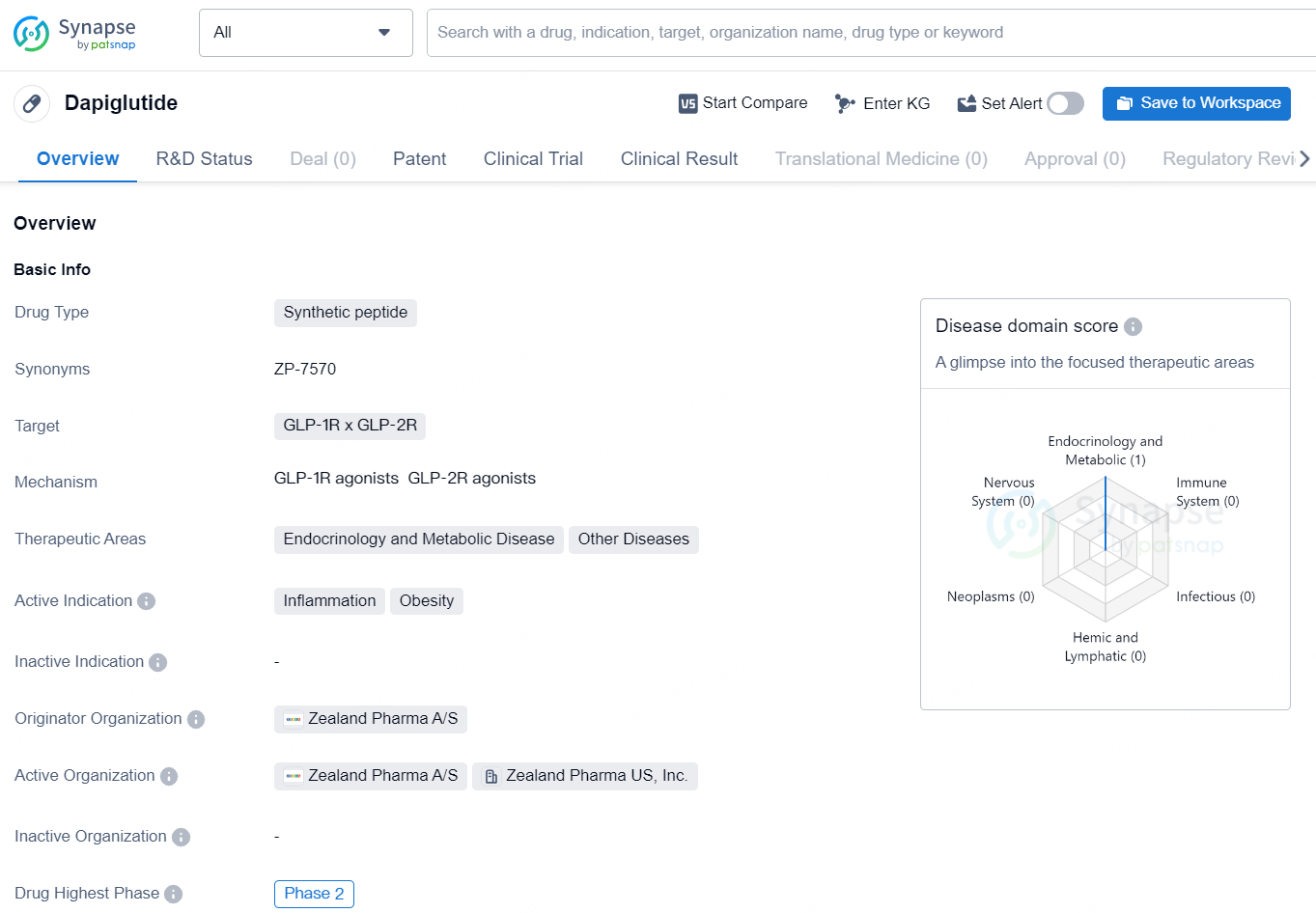
Dapiglutide is a synthetic peptide drug that targets GLP-1R x GLP-2R and is being developed for therapeutic areas including endocrinology and metabolic disease, as well as other diseases. Its active indication includes inflammation and obesity. The drug is being developed by Zealand Pharma A/S and is currently in Phase 2 of development.