Zelenirstat: A Quick Look at Its R&D Progress and Clinical Results from the 2024 ASCO_GI
Myristoylation catalyzed by N-myristoyltransferases (NMT) 1 and 2 regulates membrane-bound signaling important in cancer, including Src and Src-family tyrosine kinases. Zelenirstat is a potent oral small molecule pan-NMT inhibitor. And the latest clinical results of Zelenirstat for the treatment of relapsed/refractory advanced cancer were presented in 2024 ASCO_GI.
Zelenirstat's R&D Progress
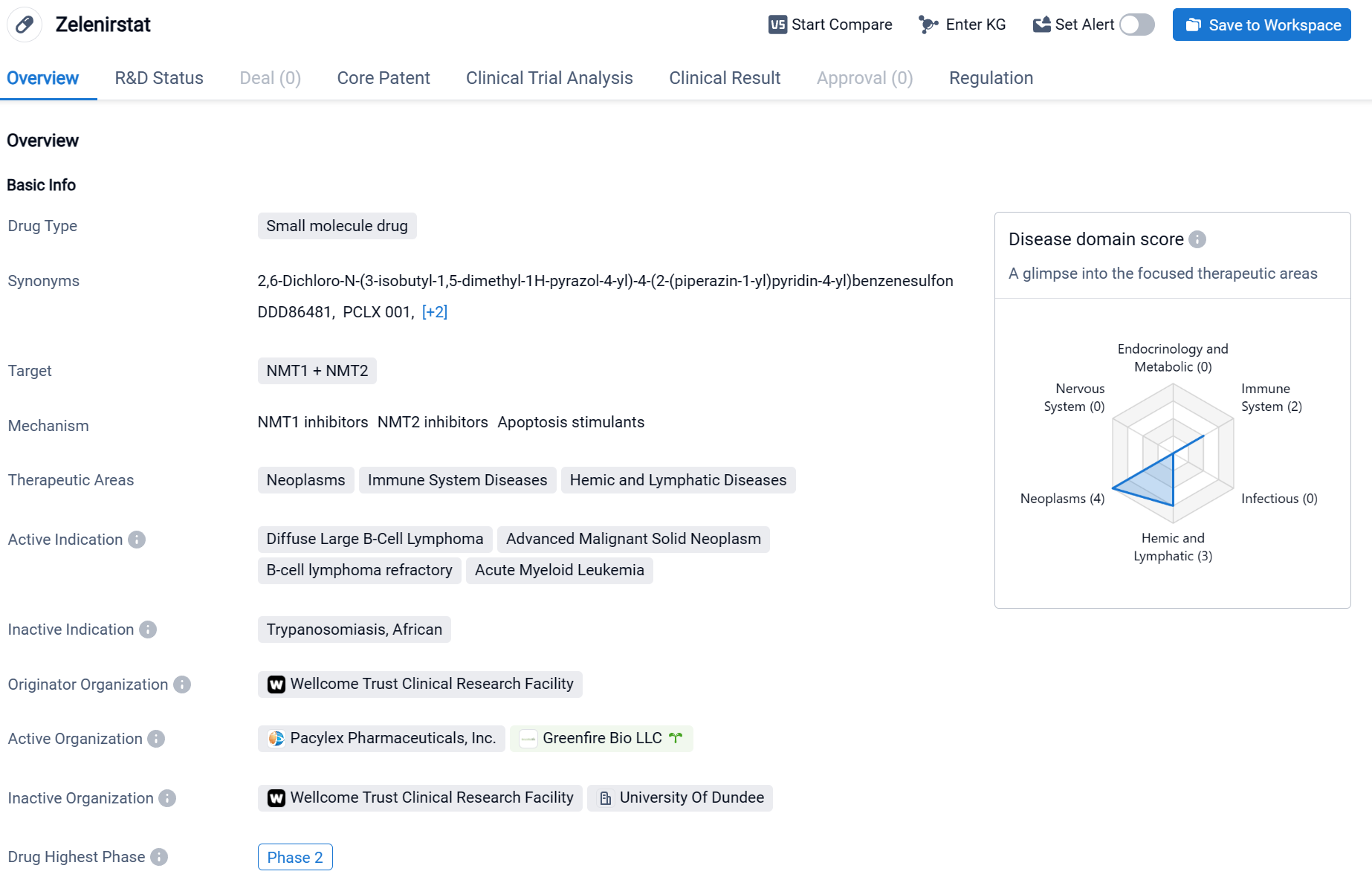
Zelenirstat is a small molecule drug that targets NMT1 and NMT2. It falls under the therapeutic areas of neoplasms, immune system diseases, and hemic and lymphatic diseases. The drug is indicated for the treatment of diffuse large B-cell lymphoma, advanced malignant solid neoplasm, B-cell lymphoma refractory, and acute myeloid leukemia.
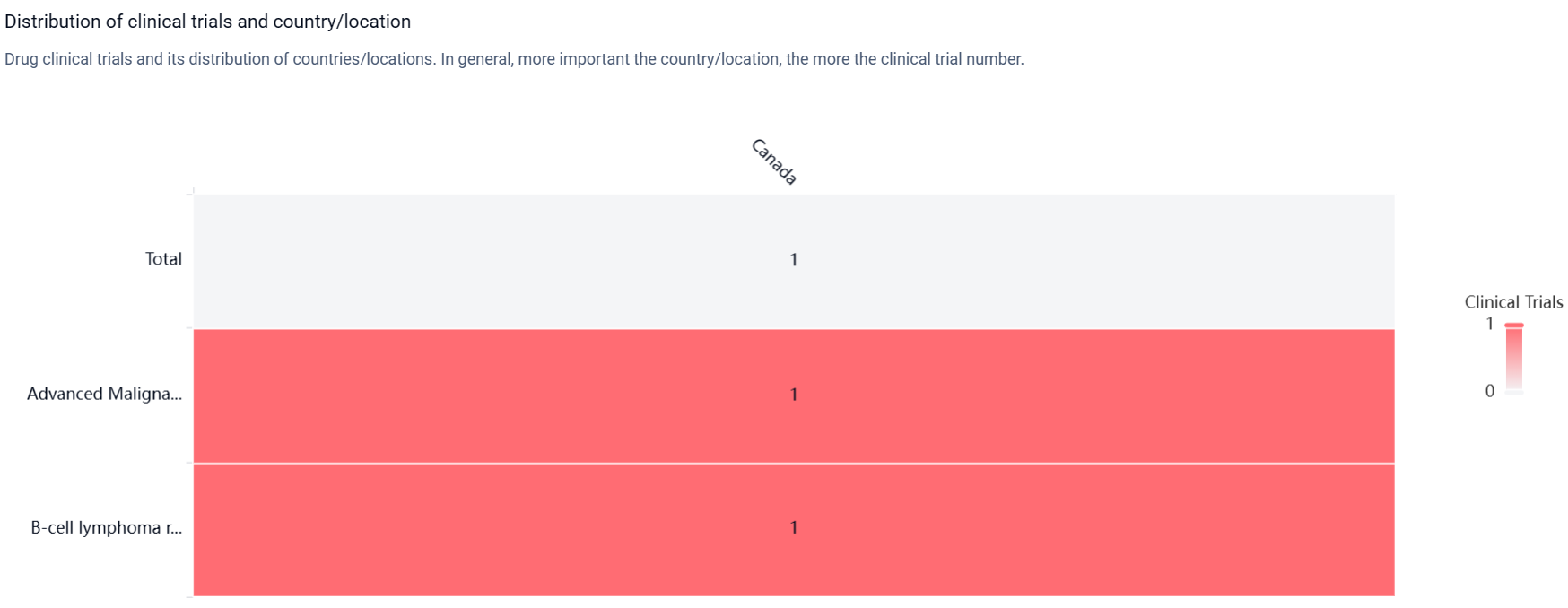
According to the Patsnap Synapse, Zelenirstat is developed by the Wellcome Trust Clinical Research Facility. Currently, the drug is in the highest phase of development, which is Phase 2. And the clinical trial distributions for Zelenirstat are primarily in Canada. The key indication is Advanced Malignant Solid Neoplasm.
Detailed Clinical Result of Zelenirstat
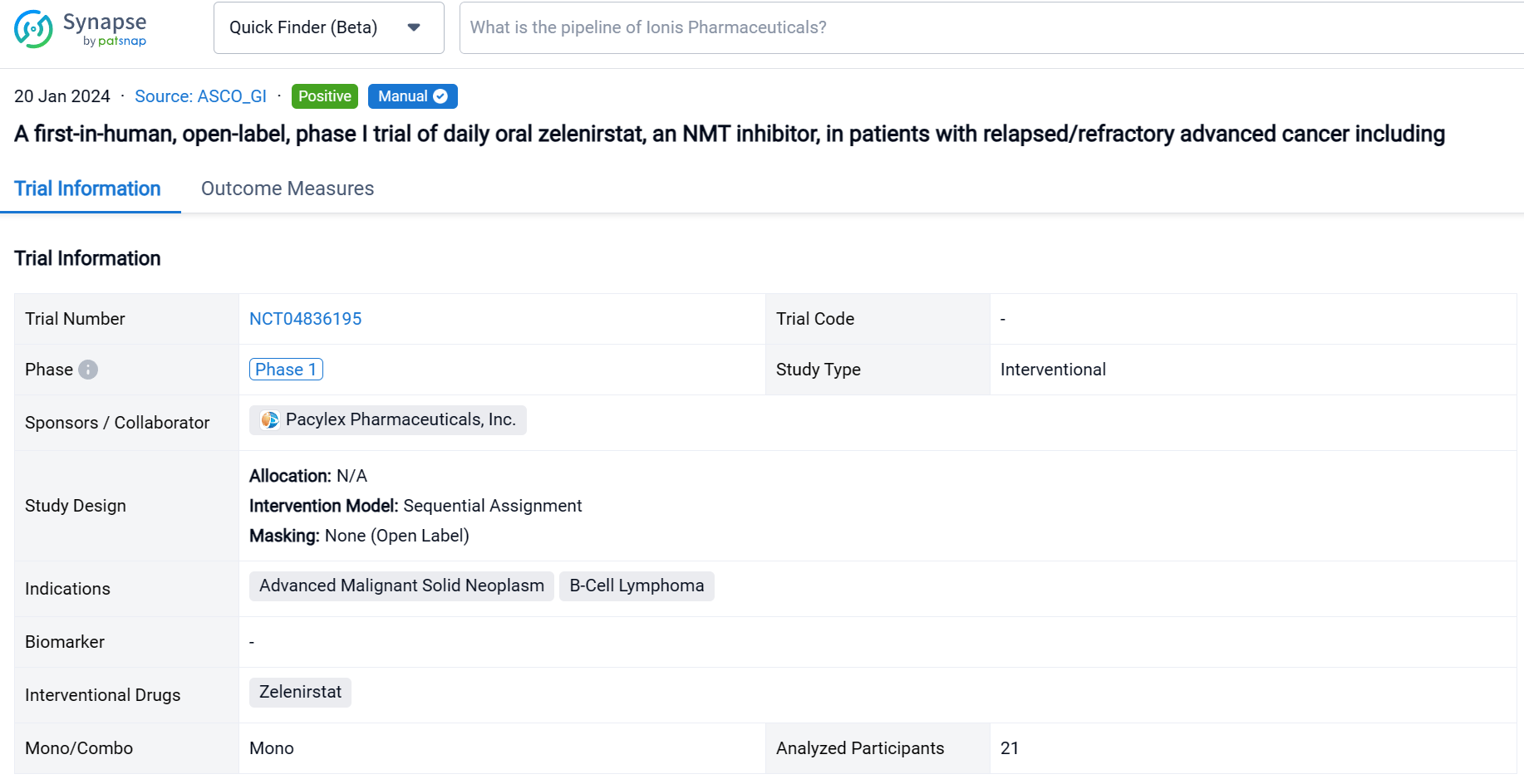
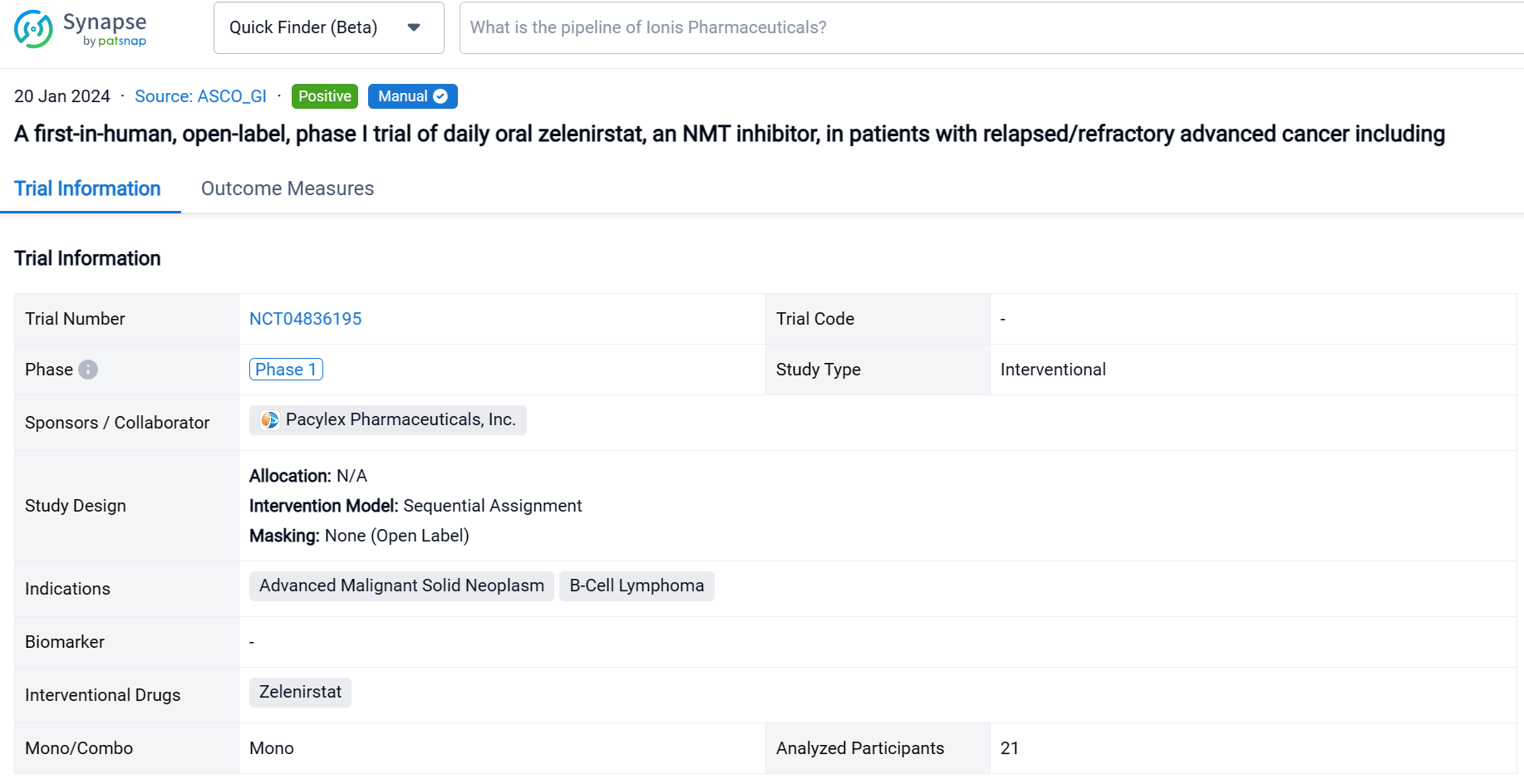
This sequential assignment, open-labeled clinical trial (NCT04836195) was aimed to determine the safety, tolerability, and maximum tolerated dose (MTD) of zelenirstat monotherapy in patients (pts) with refractory cancers.
In this study, pts with advanced solid malignancies or relapsed/refractory B-cell lymphomas were dose escalated with daily oral zelenirstat in 28-day cycles until progressive disease or dose-limiting toxicity (DLT).
The result showed that between September 2021 and July 2023, pts received 28-day cycles of zelenirstat ranging from 20 mg/d to 280 mg/d. In 21 evaluable patients, DLT was not observed up to and including the 210 mg/d cohort. Gastrointestinal DLTs were seen in the 280 mg/d cohort, establishing 210 mg/d to be the MTD. In doses up to and including 210 mg/d, gastrointestinal adverse events were the most common, reported in 29% of pts; these were primarily Gr 1 or 2 diarrhea, nausea or vomiting. Transient Gr 2 thrombocytopenia was seen in 3 pts. Zelenirstat did not induce neuropathy, alopecia, or prolong QT intervals. Plasma concentrations peaked between 1h and 4 h, terminal half-lives were 6.7 to 12 h, and steady state was achieved by day 8. Proton pump inhibitors lowered exposure and were prohibited in the higher dose cohorts. At 100 mg/d and higher, trough plasma concentrations exceeded the levels predicted to be therapeutic. Stable disease as best response was seen in 5 (30%) of 17 evaluable pts, including 1 patient with advanced refractory colon cancer who received 6 cycles of therapy at 140 mg daily dosing prior to PD. Another colon cancer patient with 5 prior lines of therapy continues 210 mg/d dosing after > 5 cycles of therapy, with non-RECIST criteria reductions of approximately 50% in CEA and tumor volumes.
It can be concluded that Zelenirstat was well-tolerated on a continuous daily oral schedule at doses as high as 210 mg/d, establishing the MTD to be 210 mg. The absence of severe toxicities, attainment of plasma concentrations highly active in preclinical models, and early evidence of clinical benefit in patients with refractory colon cancer warrant further trials.
How to Easily View the Clinical Results Using Synapse Database?
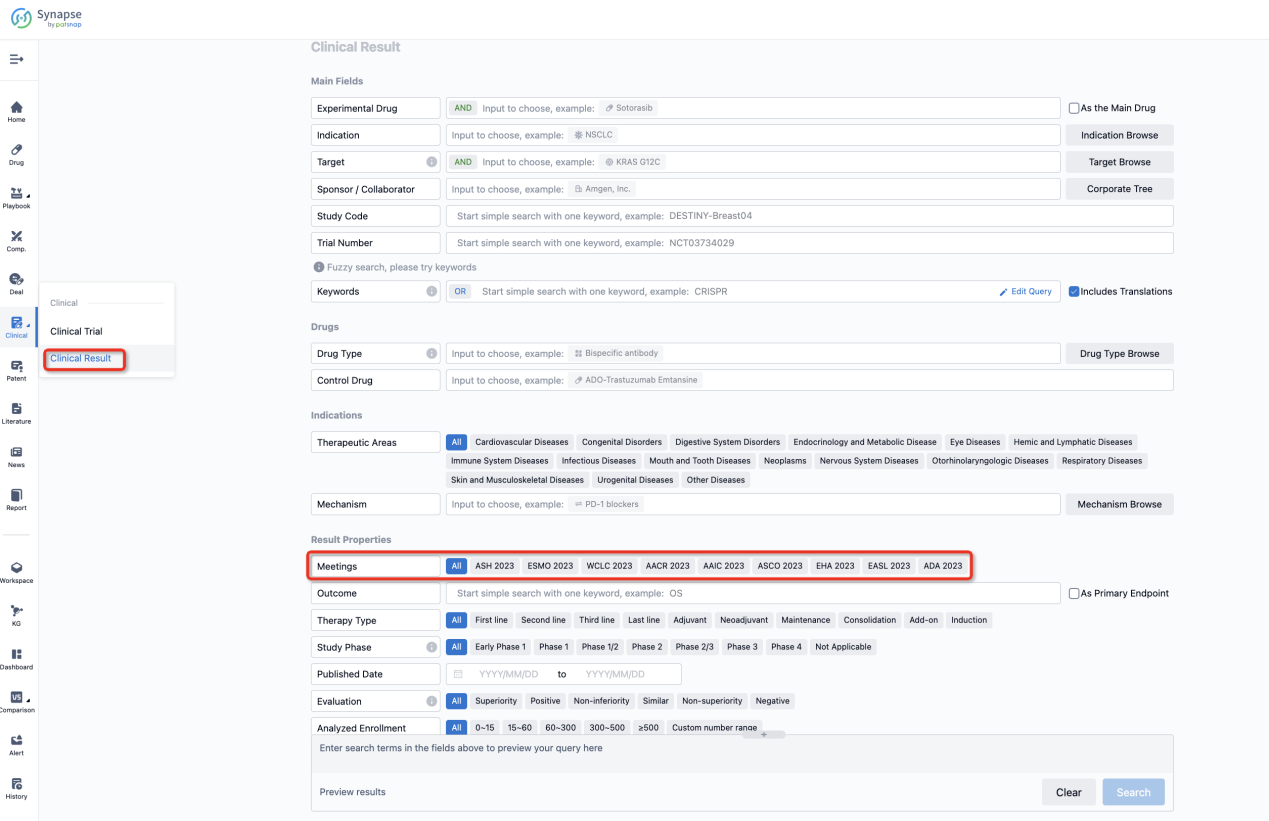
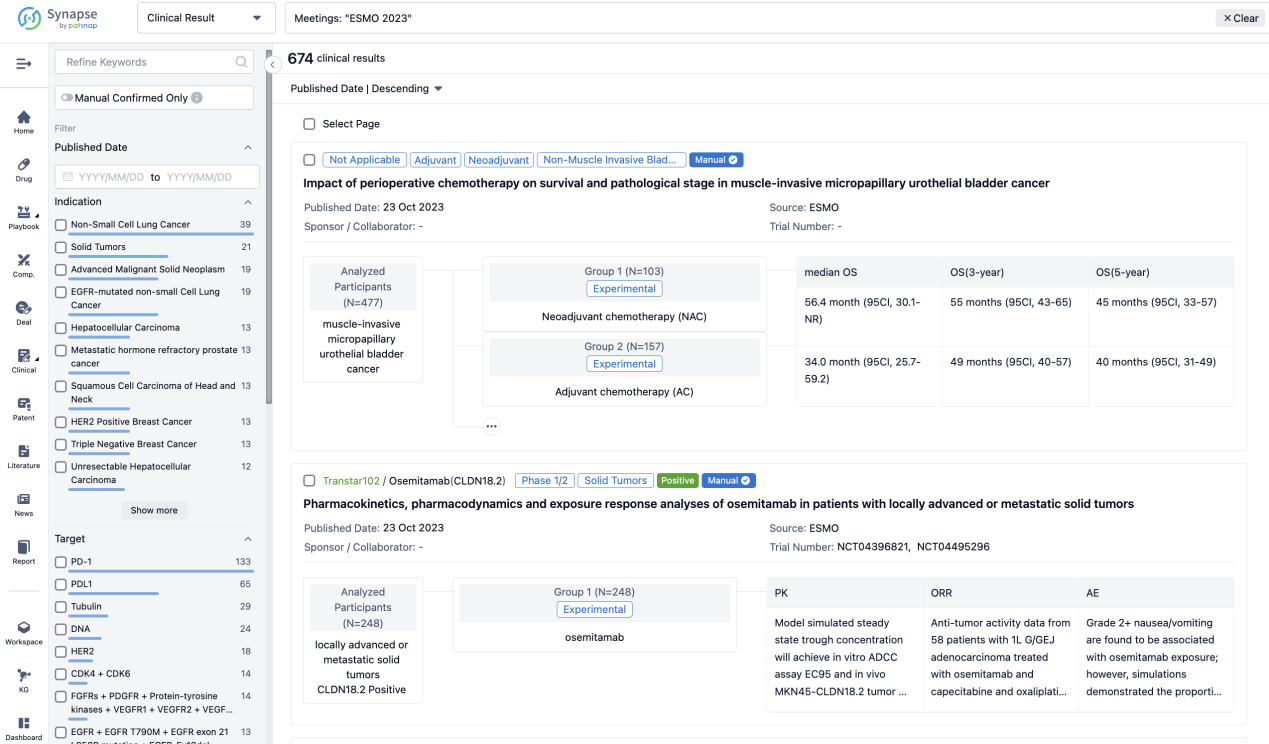
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
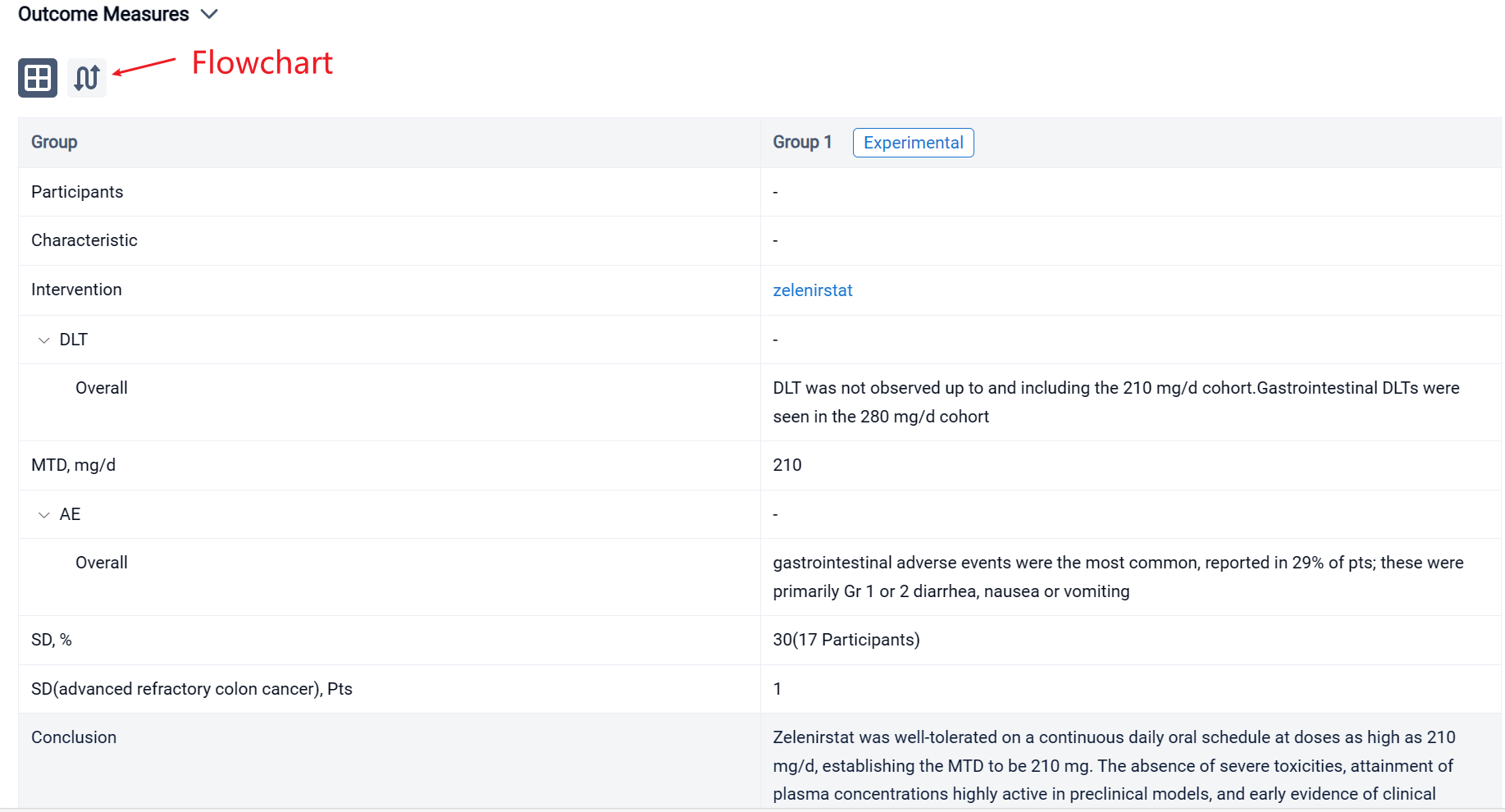
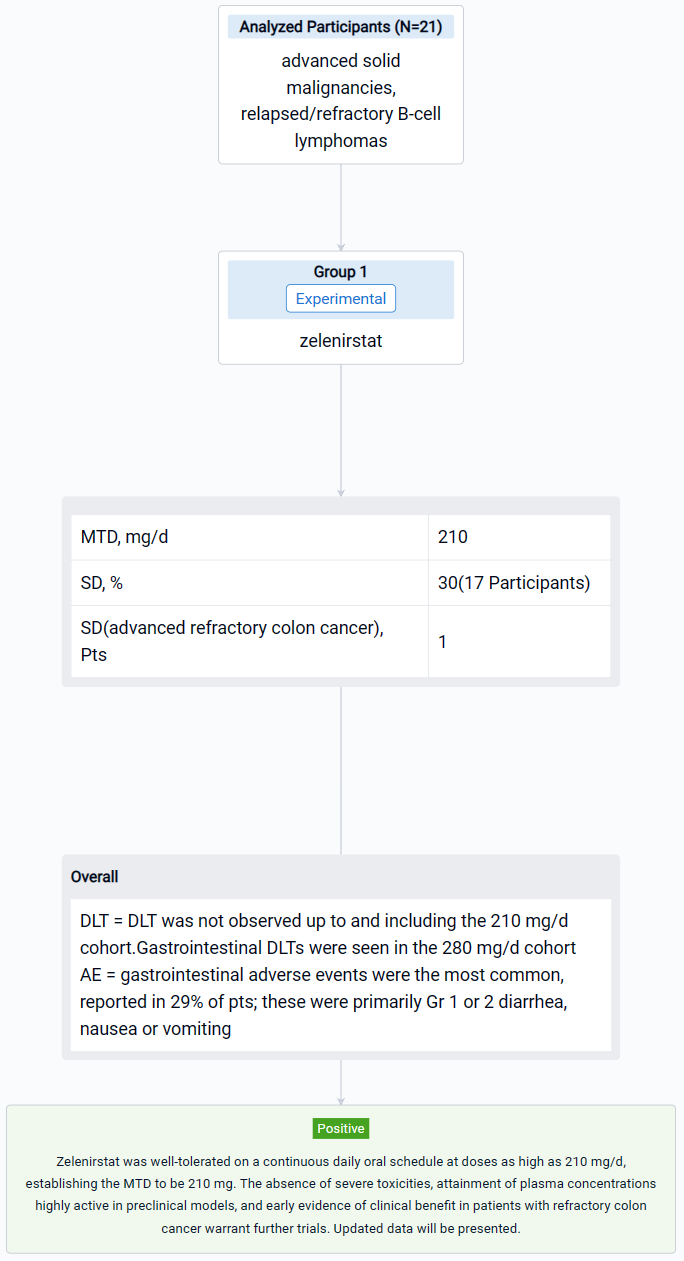
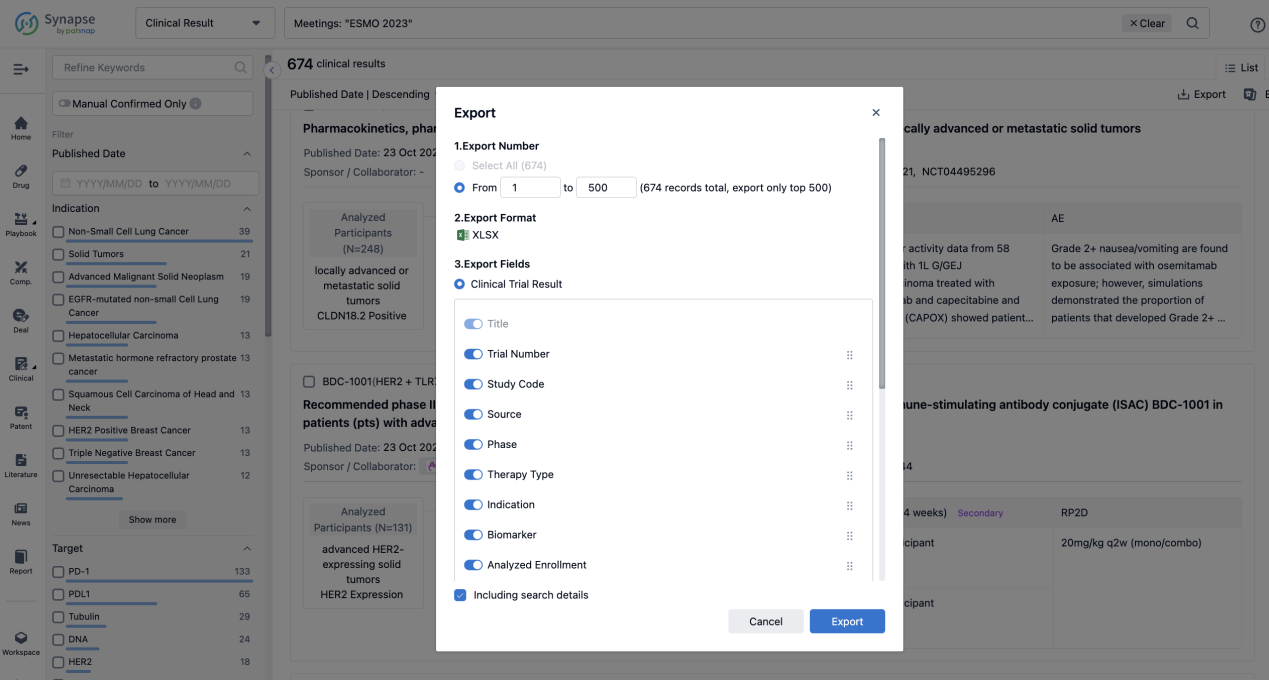
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!