Master Tretinoin Search on Synapse
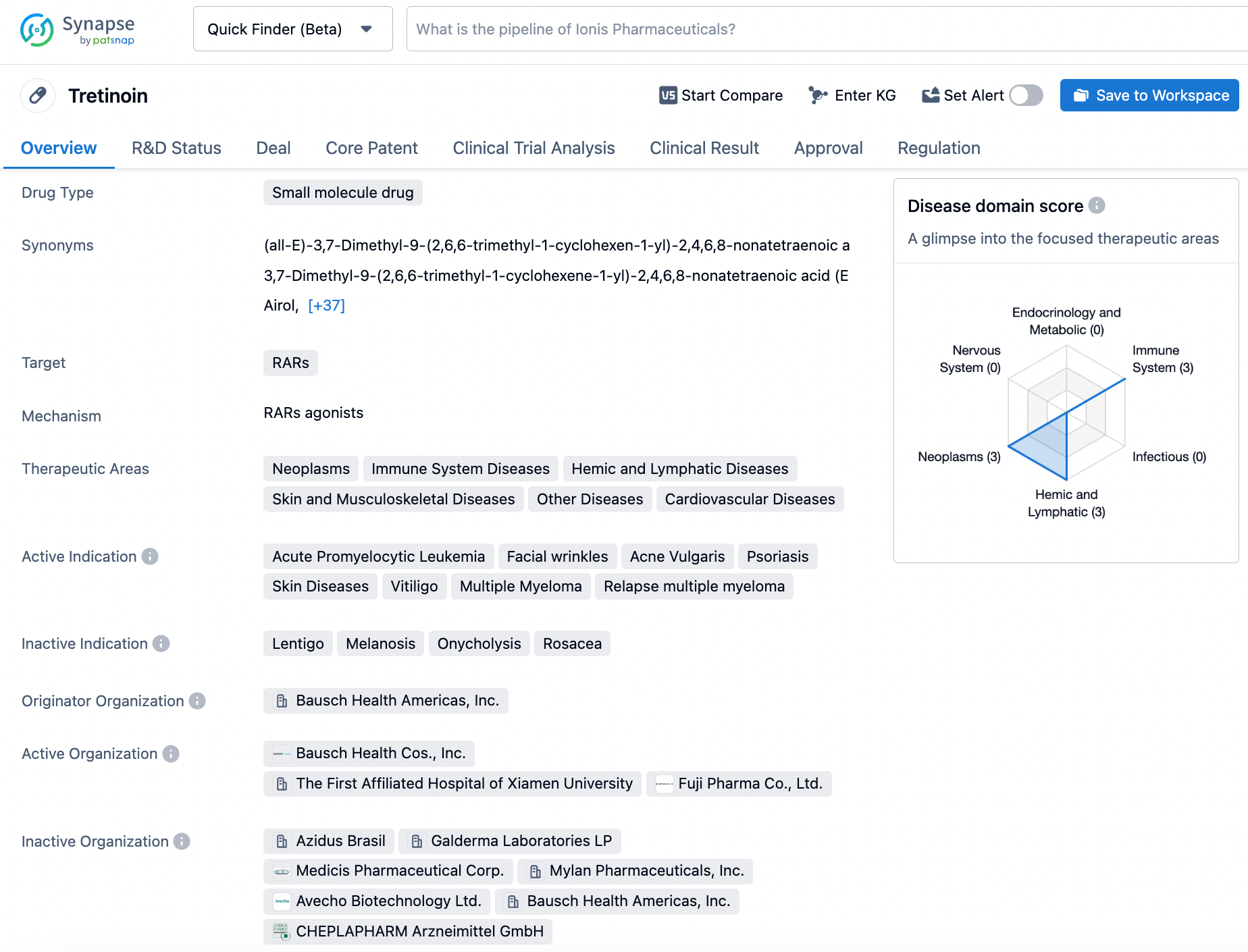
Vesanoid, also known as Tretinoin, is a vitamin A derivative that has been given the green light for treating acne vulgaris and specific forms of promyelocytic leukemia. This drug, originating from the pharmaceutical company Bausch Health Americas, acts as a RARs agonist that helps regulate gene expression and cell differentiation in a multifaceted manner, displaying a higher level of complexity in its mechanism of action. With its initial approval in the United States in 1971, Tretinoin has been increasingly incorporated into numerous over-the-counter products, due to its demonstrated efficacy in managing a wide range of skin conditions. Because of its reliability in the management of acne vulgaris, Tretinoin has gained widespread popularity among dermatologists and individuals seeking to alleviate this all-too-common skin condition. Its continued use in contemporary medicine reinforces Tretinoin's value as a valuable weapon in the struggle against acne and other cutaneous disorders, lending credence to its ongoing utility. Click on the image below to begin the exploration journey of Tretinoin through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!