Request Demo
Last update 08 May 2025
Relapsing Fever
Last update 08 May 2025
Basic Info
Synonyms FEVER, FAMINE, Fever, Relapsing, Fever;relapsing + [30] |
Introduction An acute infection characterized by recurrent episodes of PYREXIA alternating with asymptomatic intervals of apparent recovery. This condition is caused by SPIROCHETES of the genus BORRELIA. It is transmitted by the BITES of either the body louse (PEDICULUS humanus corporis), for which humans are the reservoir, or by soft ticks of the genus ORNITHODOROS, for which rodents and other animals are the principal reservoirs. |
Related
3
Drugs associated with Relapsing FeverTarget |
Mechanism 30S subunit inhibitors |
Active Org. |
Originator Org. |
Inactive Indication |
Drug Highest PhaseApproved |
First Approval Ctry. / Loc. United States |
First Approval Date05 Dec 1967 |
Target |
Mechanism 30S subunit inhibitors [+1] |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication- |
Drug Highest PhaseApproved |
First Approval Ctry. / Loc. United States |
First Approval Date08 Oct 1957 |
Target |
Mechanism IL-1β inhibitors |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication |
Drug Highest PhasePhase 3 |
First Approval Ctry. / Loc.- |
First Approval Date20 Jan 1800 |
12
Clinical Trials associated with Relapsing FeverNCT06045481
Postexposure Prophylaxis With Single Dose Doxycycline for the Prevention of Tick-borne Relapsing Fever
The goal of this clinical trial is to compare standard treatment (5 days of doxycycline) vs single dose doxycycline for the prevention of tick-borne relapsing fever in soldiers who found bite marks on their bodies after an activity that includes contact with the ground or staying at a site suspected of being infected with ticks. The main question[s] it aims to answer are:
Testing whether preventive treatment with a single dose of doxylin at a dose of 200 mg is effective in preventing recurrent fever, and if so, at what rate
Checking the profile and rate of side effects in each one of the proposed treatment protocols Participants will be treated with standard treatment (5 days of doxycycline) or single dose doxycycline.
Testing whether preventive treatment with a single dose of doxylin at a dose of 200 mg is effective in preventing recurrent fever, and if so, at what rate
Checking the profile and rate of side effects in each one of the proposed treatment protocols Participants will be treated with standard treatment (5 days of doxycycline) or single dose doxycycline.
Start Date01 Aug 2024 |
Sponsor / Collaborator- |
CTR20242238
盐酸多西环素片人体生物等效性研究
[Translation] Study on the bioequivalence of doxycycline hydrochloride tablets in healthy volunteers
以中国健康受试者为试验对象,采用自身交叉对照的试验设计,测定德全药品(江苏)股份有限公司持证生产的盐酸多西环素片给药后血浆中的多西环素在健康受试者体内的血药浓度经时过程,估算相应的药代动力学参数,并以PfizerJapanInc.持证生产的盐酸多西环素片[商品名:Vibramycin,规格:100mg(按C22H24NO8计)]为参比,评价制剂间的生物等效性,并观察盐酸多西环素片在中国健康受试者中的安全性。
[Translation]
Healthy Chinese subjects were used as test subjects. A self-crossover design was used to determine the time course of doxycycline concentration in plasma after administration of doxycycline hydrochloride tablets produced by Dequan Pharmaceutical (Jiangsu) Co., Ltd., and to estimate the corresponding pharmacokinetic parameters. Doxycycline hydrochloride tablets produced by Pfizer Japan Inc. under a license [trade name: Vibramycin, specification: 100 mg (calculated as C22H24NO8)] were used as reference to evaluate the bioequivalence between the preparations and to observe the safety of doxycycline hydrochloride tablets in healthy Chinese subjects.
Start Date18 Jul 2024 |
Sponsor / Collaborator |
CTR20234185
盐酸多西环素片在健康受试者中随机、开放、两制剂、单次给药、空腹和餐后状态下的生物等效性研究
[Translation] A randomized, open-label, two-dose, single-dose, fasting and fed bioequivalence study of doxycycline hydrochloride tablets in healthy subjects
主要目的:研究空腹及餐后状态下单次口服受试制剂盐酸多西环素片(规格:0.1g,杭州绿哲医药科技有限公司持证)与参比制剂盐酸多西环素片(商品名:Vibramycin®,规格:100mg,持证商:Pfizer Japan Inc.)在健康成年受试者体内的药代动力学,评价空腹及餐后状态口服两种制剂的生物等效性。
[Translation]
Main objective: To study the pharmacokinetics of the test preparation doxycycline hydrochloride tablets (specification: 0.1g, licensed by Hangzhou Lvzhe Pharmaceutical Technology Co., Ltd.) and the reference preparation doxycycline hydrochloride tablets (trade name: Vibramycin®, specification: 100mg, licensed by Pfizer Japan Inc.) in healthy adult subjects after a single oral administration in the fasting and fed state, and to evaluate the bioequivalence of the two preparations when taken orally in the fasting and fed state.
Start Date11 Jan 2024 |
Sponsor / Collaborator |
100 Clinical Results associated with Relapsing Fever
Login to view more data
100 Translational Medicine associated with Relapsing Fever
Login to view more data
0 Patents (Medical) associated with Relapsing Fever
Login to view more data
4,020
Literatures (Medical) associated with Relapsing Fever01 Dec 2025·Journal of Clinical Immunology
Non-Skewed X-inactivation Results in NF-κB Essential Modulator (NEMO) Δ-exon 5-autoinflammatory Syndrome (NEMO-NDAS) in a Female with Incontinentia Pigmenti
Article
Author: Pannicke, Ulrich ; Lee-Kirsch, Min Ae ; von Bernuth, Horst ; Schwarz, Klaus ; Hoenig, Manfred ; Furlan, Ingrid ; Schulz, Ansgar ; Führer, Marita ; Debatin, Klaus-Michael ; Zinngrebe, Julia ; Lorenz, Myriam ; Drewes, Cosima ; Felgentreff, Kerstin ; Peters, Sarah ; Eigemann, Jessica ; Siebert, Reiner ; Kölsch, Uwe ; Janda, Ales ; Jacobsen, Eva-Maria ; Schuetz, Catharina ; Rump, Eva-Maria
01 Dec 2025·Journal of Clinical Immunology
Development of an Expert-Based Scoring System for Early Identification of Patients with Inborn Errors of Immunity in Primary Care Settings – the PIDCAP Project
Article
Author: Piera-Jiménez, Jordi ; Soler-Palacin, Pere ; Cos, Xavier ; de la Torre, Sergi ; Carot-Sans, Gerard ; Serra-Picamal, Xavier ; Rivière, Jacques G
01 Aug 2025·American Journal of Ophthalmology
Ocular Features of VEXAS Syndrome: A Systematic Review and Meta-analysis
Review
Author: Pietris, James ; Selva, Dinesh ; Zgaga, Lina ; Ang, Terence ; Quigley, Clare
6
News (Medical) associated with Relapsing Fever11 Jun 2024
With the tafenoquine for acute babesiosis orphan drug designation, 60 Degrees Pharmaceuticals now qualifies for certain incentives, including market exclusivity, tax credits, and exemption from certain FDA filing fees.60 Degrees Pharmaceuticals recently announced it has entered into an agreement with Tufts Medical Center in Boston to conduct the world’s first clinical trial evaluating the efficacy and safety of tafenoquine in treating human babesiosis patients.FDA orphan drug designation is granted for therapeutic candidates that may prevent or treat a rare disease or condition, such as acute babesiosis. WASHINGTON, June 11, 2024 (GLOBE NEWSWIRE) -- 60 Degrees Pharmaceuticals, Inc. (NASDAQ: SXTP; SXTPW) (the “Company” or “60 Degrees Pharmaceuticals”), a pharmaceutical company focused on developing new medicines for infectious diseases, announced today that the U.S. Food and Drug Administration (“FDA”) has granted its investigational tafenoquine candidate orphan drug designation for the treatment of patients with acute babesiosis. FDA orphan drug designation is granted for therapeutic candidates that may prevent or treat a rare disease or condition, such as acute babesiosis. Babesiosis is a steadily emerging, infectious disease transmitted by a microscopic parasite, Babesia, through the bite of the black-legged (deer) tick, the vector that spreads Lyme disease. Babesiosis may be life-threatening in elderly and immunosuppressed patients. Up to 10 percent of Lyme disease patients may be coinfected with Babesia. Therefore, up to 47,600 of the estimated 476,000 patients with new Lyme infections each year may be co-infected with Babesia. “Results of recent animal studies of tafenoquine show exciting promise for the drug to have potential in human patients with acute babesiosis,” said Chief Executive Officer of 60 Degrees Pharmaceuticals, Geoff Dow, PhD. “The FDA granting tafenoquine orphan drug designation for acute babesiosis validates the growing need for an additional therapeutic option that infectious disease specialists can use in addressing this very serious, potentially life-threatening illness. We look forward to results of our clinical trial program in coming months and to the prospect of securing a secondary indication for tafenoquine in the area of acute babesiosis treatment.” 60 Degrees Pharmaceuticals recently announced it has entered into an agreement with Tufts Medical Center in Boston to conduct the world’s first clinical trial evaluating the efficacy and safety of tafenoquine in treating patients who have acute babesiosis. Recruitment for the trial will begin on June 13, 2024, and will include at least 24 patients hospitalized with babesiosis. Additional recruitment sites at prominent university hospitals in the Northeast U.S. are also planned. With the tafenoquine for acute babesiosis orphan drug designation, 60 Degrees Pharmaceuticals now qualifies for certain incentives, including market exclusivity, tax credits, and exemption from certain FDA filing fees. About Tafenoquine Tafenoquine is approved for malaria prophylaxis in the United States under the product name ARAKODA®. The safety of the approved regimen of tafenoquine for malaria prophylaxis has been assessed in five separate randomized, double-blind, active comparator or placebo-controlled trials for durations of up to six months. Tafenoquine has not been proven to be effective for treatment or prevention of babesiosis and is not approved by the U.S. Food and Drug Administration for such an indication. About the Study of Tafenoquine for Patients Hospitalized with Babesiosis The study is a randomized, double-blind, placebo-controlled trial that will enroll patients at multiple sites in the Northeast U.S. and will compare the safety and efficacy of tafenoquine versus placebo in patients hospitalized for babesiosis with low risk for relapsing disease who will also be administered a standard-of-care antimicrobial regimen. The two main study endpoints will be the time to sustained clinical resolution of symptoms and the time to molecular cure as determined by an FDA-approved nucleic acid test. At least 24, and as many as 33 patients, will be recruited before an interim analysis is conducted. Sufficient enrollment capacity is planned to allow all study subjects to be recruited during the 2024 tick season (June to September) if caseload is high. The interim analysis will include both a test of significance, as well as size re-estimation to allow additional recruitment if required. The study will be conducted at three hospitals in the Northeast U.S. The efficacy and safety of 8-aminoquinolines, a class of drugs that includes tafenoquine and primaquine, for prevention and treatment of malaria is well documented. Several case reports of tafenoquine use for babesiosis indicate that the drug is already being used for this purpose in the practice of medicine in the U.S. About ARAKODA® (tafenoquine)Tafenoquine was discovered by Walter Reed Army Institute of Research. Tafenoquine was approved for malaria prophylaxis in 2018 in the United States as ARAKODA® and in Australia as KODATEF®. Both were commercially launched in 2019 and are currently distributed through pharmaceutical wholesaler networks in each respective country. They are available at retail pharmacies as a prescription-only malaria prevention drug. According to the Centers for Disease Control and Prevention, the long terminal half-life of tafenoquine, which is approximately 16 days, may offer potential advantages in less-frequent dosing for prophylaxis for malaria. ARAKODA® is not suitable for everyone, and patients and prescribers should review the Important Safety Information below. Individuals at risk of contracting malaria are prescribed ARAKODA® 2 x 100 mg tablets once per day for three days (the loading phase) prior to travel to an area of the world where malaria is endemic, 2 x 100 mg tablets weekly for up to six months during travel, then 2 x 100 mg in the week following travel. ARAKODA® (tafenoquine) Important Safety InformationARAKODA® is an antimalarial indicated for the prophylaxis of malaria in patients aged 18 years of age and older. ContraindicationsARAKODA® should not be administered to: Glucose-6-phosphate dehydrogenase (“G6PD”) deficiency or unknown G6PD status;Breastfeeding by a lactating woman when the infant is found to be G6PD deficient; or ifG6PD status is unknown;Patients with a history of psychotic disorders or current psychotic symptoms; orKnown hypersensitivity reactions to tafenoquine, other 8-aminoquinolines, or any component of ARAKODA®. Warnings and PrecautionsHemolytic Anemia: G6PD testing must be performed before prescribing ARAKODA® due to the risk of hemolytic anemia. Monitor patients for signs or symptoms of hemolysis.G6PD Deficiency in Pregnancy or Lactation: ARAKODA® may cause fetal harm when administered to a pregnant woman with a G6PD-deficient fetus. ARAKODA® is not recommended during pregnancy. A G6PD-deficient infant may be at risk for hemolytic anemia from exposure to ARAKODA® through breast milk. Check infant’s G6PD status before breastfeeding begins.Methemoglobinemia: Asymptomatic elevations in blood methemoglobin have been observed. Initiate appropriate therapy if signs or symptoms of methemoglobinemia occur.Psychiatric Effects: Serious psychotic adverse reactions have been observed in patients with a history of psychosis or schizophrenia, at doses different from the approved dose. If psychotic symptoms (hallucinations, delusions, or grossly disorganized thinking or behavior) occur, consider discontinuation of ARAKODA® therapy and evaluation by a mental health professional as soon as possible.Hypersensitivity Reactions: Serious hypersensitivity reactions have been observed with administration of ARAKODA®. If hypersensitivity reactions occur, institute appropriate therapy.Delayed Adverse Reactions: Due to the long half-life of ARAKODA® (approximately 16 days), psychiatric effects, hemolytic anemia, methemoglobinemia, and hypersensitivity reactions may be delayed in onset and/or duration.Adverse Reactions: The most common adverse reactions (incidence greater than or equal to 1 percent) were: headache, dizziness, back pain, diarrhea, nausea, vomiting, increased alanine aminotransferase, motion sickness, insomnia, depression, abnormal dreams, and anxiety.Drug InteractionsAvoid co-administration with drugs that are substrates of organic cation transporter-2 or multidrug and toxin extrusion transporters. Use in Specific PopulationsLactation: Advise women not to breastfeed a G6PD-deficient infant or infant with unknown G6PD status during treatment and for 3 months after the last dose of ARAKODA®.To report SUSPECTED ADVERSE REACTIONS, contact 60 Degrees Pharmaceuticals, Inc. at 1- 888-834-0225 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. The full prescribing information of ARAKODA® is located here. About 60 Degrees Pharmaceuticals, Inc.60 Degrees Pharmaceuticals, Inc., founded in 2010, specializes in developing and marketing new medicines for the treatment and prevention of infectious diseases that affect the lives of millions of people. 60 Degrees Pharmaceuticals, Inc. achieved FDA approval of its lead product, ARAKODA® (tafenoquine), for malaria prevention, in 2018. 60 Degrees Pharmaceuticals, Inc. also collaborates with prominent research organizations in the U.S., Australia, and Singapore. The 60 Degrees Pharmaceuticals, Inc. mission has been supported through in-kind funding from the U.S. Department of Defense and private institutional investors including Knight Therapeutics Inc., a Canadian-based pan-American specialty pharmaceutical company. 60 Degrees Pharmaceuticals, Inc. is headquartered in Washington D.C., with a majority-owned subsidiary in Australia. Learn more at www.60degreespharma.com. The statements contained herein may include prospects, statements of future expectations and other forward-looking statements that are based on management’s current views and assumptions and involve known and unknown risks and uncertainties. Actual results, performance or events may differ materially from those expressed or implied in such forward-looking statements. Cautionary Note Regarding Forward-Looking StatementsThis press release may contain “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward‐looking statements reflect the current view about future events. When used in this press release, the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan,” or the negative of these terms and similar expressions, as they relate to us or our management, identify forward‐looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy, activities of regulators and future regulations and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: there is substantial doubt as to our ability to continue on a going-concern basis; we might not be eligible for Australian government research and development tax rebates; if we are not able to successfully develop, obtain FDA approval for, and provide for the commercialization of non- malaria prevention indications for tafenoquine (ARAKODA® or other regimen) or Celgosivir in a timely manner, we may not be able to expand our business operations; we may not be able to successfully conduct planned clinical trials or patient recruitment in our trials might be slow or negligible; and we have no manufacturing capacity which puts us at risk of lengthy and costly delays of bringing our products to market. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company’s filings with the Securities and Exchange Commission (“SEC”), including the information contained in our Annual Report on Form 10-K filed with the SEC on April 1, 2024, and our subsequent SEC filings. Investors and security holders are urged to read these documents free of charge on the SEC’s web site at www.sec.gov. As a result of these matters, changes in facts, assumptions not being realized or other circumstances, the Company’s actual results may differ materially from the expected results discussed in the forward-looking statements contained in this press release. Any forward-looking statement made by us in this press release is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. Media Contact:Sheila A. BurkeSheilaBurke-consultant@60degreespharma.com(484) 667-6330 Investor Contact:Patrick Gaynespatrickgaynes@60degreespharma.com(310) 989-5666
Drug ApprovalOrphan DrugClinical Study
21 Jul 2023
THURSDAY, July 20, 2023 -- During 2012 to 2021, 251 cases of soft tick relapsing fever (STRF), caused by certain
Borrelia
spirochetes and transmitted to humans by soft-bodied
Ornithodoros
ticks, were reported in 11 states, according to research published in the July 21 issue of the U.S. Centers for Disease Control and Prevention
Morbidity and Mortality Weekly Report
.
Amy M. Beeson, M.D., from the CDC in Atlanta, and colleagues summarized demographic and clinical information for STRF cases reported during 2012 to 2021.
The researchers identified 251 cases in 11 states during the study period, with a median annual case count of 24. During the study period, there was no significant change in the number of cases observed. Overall, 55 percent of the patients with STRF were hospitalized, but there were no fatalities. Since the 1990s, the geographic distribution and seasonal pattern of STRF have remained relatively constant. In areas where STRF is endemic, persons should avoid rodent-infested structures and habitats, such as caves.
"To reduce STRF incidence in the United States, progress in surveillance, prevention, and disease recognition is needed," the authors write. "Residents and visitors to areas where STRF is endemic should be educated about how to prevent soft tick bites (most importantly, avoidance of rodent-infested structures and rodent habitats such as caves) and when to seek medical care."
Abstract/Full Text
Posted July 2023
Clinical Result
11 Nov 2022
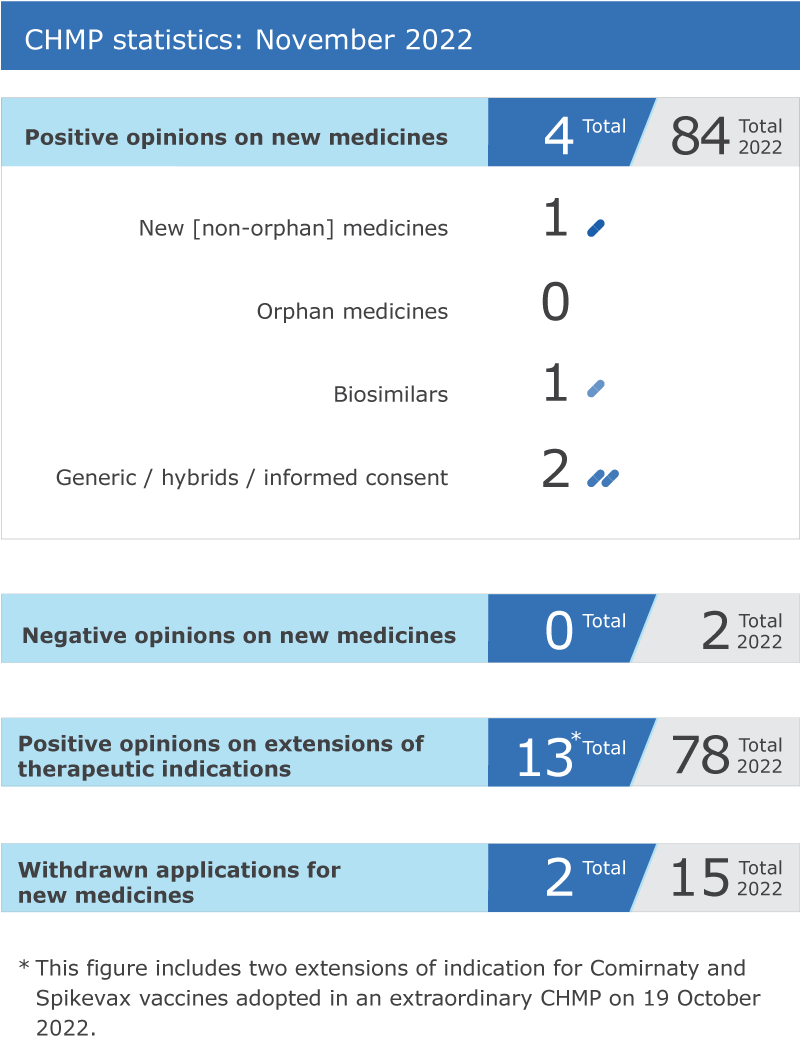
Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 7-10 November 2022
Four new medicines recommended for approval
EMA’s human medicines committee (
CHMP
) recommended four medicines for approval at its November 2022 meeting.
The
CHMP
recommended authorising the COVID-19 vaccine
VidPrevtynBeta
(COVID-19 vaccine (recombinant, adjuvanted)) as a booster in adults previously vaccinated with an mRNA or adenoviral vector COVID-19 vaccine. It is the seventh vaccine recommended in the European Union (EU) for protecting against COVID-19 and, together with the vaccines already authorised, will support vaccination campaigns in EU Member States during the pandemic. See more information in the news announcement in the grid below.
The committee adopted a positive opinion for a
biosimilar medicine
,
Kauliv
(teriparatide), for the treatment of osteoporosis, a health condition that weakens bones, making them fragile and more likely to break.
A
generic medicine
,
Pirfenidone Viatris
(pirfenidone), received a positive opinion for the treatment of idiopathic pulmonary fibrosis, a chronic and progressive condition in which the lungs become scarred and breathing becomes increasingly difficult.
The
CHMP
adopted a positive opinion for a
generic medicine
,
Sugammadex Amomed
(sugammadex), intended for the reversal of neuromuscular blockade induced by rocuronium in adults and children or vecuronium in adults. Rocuronium and vecuronium are muscle relaxants used during some types of surgeries. Sugammadex is used to speed up the recovery from the effects of the muscle relaxant.
Recommendations on extensions of therapeutic indication for 11 medicines
The committee recommended 11 extensions of
indication
for medicines that are already authorised in the EU:
Ceprotin
,
Comirnaty
,
DuoPlavin
,
Dupixent
,
Enhertu
,
Eylea
,
Imfinzi
,
Iscover
,
Lynparza
,
Plavix
and
Xofluza
.
Withdrawals of applications
Two applications for
marketing authorisation
were withdrawn:
Orepaxam
* for the treatment of pulmonary arterial hypertension, and
Febseltiq
* for the treatment of cholangiocarcinoma (cancer of the bile ducts).
Two applications for extensions of therapeutic
indications
were withdrawn:
Gavreto
for the treatment of certain types of thyroid cancer, and
Ilaris
for the treatment of Schnitzler syndrome, a rare inflammatory disease causing long-term urticaria, recurrent fever, bone and joint pain, and swollen lymph nodes.
Question-and-answer documents on the withdrawals are available in the grid below.
COVID-19 update
The committee recommended extending the use of COVID-19 vaccine
Comirnaty
targeting the original strain and Omicron subvariants BA.4 and BA.5 in children between 5 to 11 years of age.
An overview of all the COVID-19 vaccines
authorised in the EU is available on EMA’s website.
Safety update
The
CHMP
endorsed the measures recommended by the
Pharmacovigilance Risk Assessment Committee
(
PRAC
) to minimise the risk of serious side effects with
Janus kinase (JAK) inhibitors
used to treat several chronic inflammatory disorders. These side effects include cardiovascular conditions, blood clots, cancer and serious infections. This recommendation is the outcome of an article 20
referral
procedure, which is triggered for medicines that have been authorised via the
centralised procedure
in case ofquality, safety or
efficacy
issues. A public health communication on this
referral
is available in the grid below.
Agenda and minutes
The agenda of the November 2022
CHMP
meeting is published on EMA's website. Minutes of the October 2022
CHMP
meeting will be published in the coming weeks.
CHMP statistics
Key figures from the November 2022
CHMP
meeting are represented in the graphic below.
*This product was designated as an
orphan medicine
during its development.
Orphan designations
are reviewed by EMA's
Committee for Orphan Medicinal Products
(
COMP
) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of
market exclusivity
.
Positive recommendation on new medicines
Name of medicine
VidPrevtyn Beta
International non-proprietary name
(INN)
COVID-19 vaccine (recombinant, adjuvanted)
Marketing-authorisation applicant
Sanofi Pasteur
Therapeutic
indication
VidPrevtyn Beta is indicated as a booster for active immunisation to prevent COVID-19 in adults who have previously received a mRNA or adenoviral vector COVID-19 vaccine
More information
VidPrevtyn Beta: Pending EC decision
News announcement:
EMA recommends approval of VidPrevtyn Beta as a COVID 19 booster vaccine
Positive recommendation on new biosimilar medicine
Name of medicine
Kauliv
INN
teriparatide
Marketing-authorisation applicant
Strides Pharma Cyprus
Therapeutic
indication
Treatment of osteoporosis
More information
Kauliv: Pending EC decision
Positive recommendations on new generic medicines
Name of medicine
Pirfenidone Viatris
INN
pirfenidone
Marketing-authorisation applicant
Viatris Limited
Therapeutic
indication
Treatment of idiopathic pulmonary fibrosis
More information
Pirfenidone Viatris: Pending EC decision
Name of medicine
Sugammadex Amomed
INN
sugammadex
Marketing-authorisation applicant
AOP Orphan Pharmaceuticals GmbH
Therapeutic
indication
Reversal of neuromuscular blockade induced by rocuronium or vecuronium
More information
Sugammadex Amomed: Pending EC decision
Positive recommendations on extensions of indications
Name of medicine
Ceprotin
INN
human protein C
Marketing-authorisation holder
Takeda Manufacturing Austria AG
More information
Ceprotin: Pending EC decision
Name of medicine
Comirnaty
INN
tozinameran
Marketing-authorisation holder
BioNTech Manufacturing GmbH
More information
Comirnaty: Pending EC decision
Name of medicine
DuoPlavin
INN
clopidogrel / acetylsalicylic acid
Marketing-authorisationholder
Sanofi-aventis groupe
More information
DuoPlavin: Pending EC decision
Name of medicine
Dupixent
INN
dupilumab
Marketing-authorisation holder
Sanofi-aventis groupe
More information
Dupixent: Pending EC decision
Name of medicine
Enhertu
INN
trastuzumab deruxtecan
Marketing-authorisation holder
Daiichi Sankyo Europe GmbH
More information
Enhertu: Pending EC decision
Name of medicine
Eylea
INN
aflibercept
Marketing-authorisation holder
Bayer AG
More information
Eylea: Pending EC decision
Name of medicine
Imfinzi
INN
durvalumab
Marketing-authorisation holder
AstraZeneca AB
More information
Imfinzi: Pending EC decision
Name of medicine
Iscover
INN
clopidogrel
Marketing-authorisationholder
Sanofi-aventis groupe
More information
Iscover: Pending EC decision
Name of medicine
Lynparza
INN
olaparib
Marketing-authorisationholder
AstraZeneca AB
More information
Lynparza: Pending EC decision
Name of medicine
Plavix
INN
clopidogrel
Marketing-authorisationholder
Sanofi-aventis groupe
More information
Plavix: Pending EC decision
Name of medicine
Xofluza
INN
baloxavir marboxil
Marketing-authorisationholder
Roche Registration GmbH
More information
Xofluza: Pending EC decision
Withdrawal of initial marketing authorisation application
Name of medicine
Febseltiq
INN
infigratinib
Marketing-authorisation applicant
Helsinn Birex Pharmaceuticals Limited
More information
Febseltiq: Withdrawn application
Name of medicine
Orepaxam
INN
treprostinil diolamine
Marketing-authorisationapplicant
Ferrer Internacional S.A.
More information
Orepaxam: Withdrawn application
Withdrawals of post-authorisation marketing authorisation applications
Name of medicine
Gavreto
INN
pralsetinib
More information
Gavreto: Withdrawn application
Name of medicine
Ilaris
INN
canakinumab
More information
Ilaris: Withdrawn application
Conclusion of referral
Name of medicine
Janus kinase (JAK) inhibitors
More information
Janus Kinase inhibitors (JAKi)
Other updates
List item
Scientific advice and protocol assistance adopted during the CHMP meeting 7-10 November 2022
(PDF/246.92 KB)
(new)
Adopted
First published: 11/11/2022
EMA/CHMP/SAWP/872304/2022
List item
Start of Union reviews adopted during the CHMP meeting of 7-10 November 2022
(PDF/106.5 KB)
(new)
Adopted
First published: 11/11/2022
EMA/846051/2022
VaccineDrug ApprovalOrphan Drug
Analysis
Perform a panoramic analysis of this field.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free

