Last update 19 Sep 2024
Heptavalent combination vaccine(GlaxoSmithKline Plc)
Last update 19 Sep 2024
Overview
Basic Info
Drug Type Prophylactic vaccine |
Synonyms Heptavalent combination vaccine, Heptavalent conjugated vaccine + [2] |
Target- |
Mechanism Immunostimulants |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Related
4
Clinical Trials associated with Heptavalent combination vaccine(GlaxoSmithKline Plc)Immunogenicity and Safety Study of GlaxoSmithKline Biologicals' GSK2202083A Vaccine Administered as a Booster Dose in 12-18 Months Old Healthy Children
The current trial will evaluate the safety and immunogenicity of GSK Biologicals' GSK2202083A vaccine when administered as a booster dose following priming in the first year of life with the same vaccine.
This protocol posting deals with objectives & outcome measures of the booster phase. The objectives & outcome measures of the primary phase are presented in a separate protocol posting (NCT number = NCT00970307).
This protocol posting deals with objectives & outcome measures of the booster phase. The objectives & outcome measures of the primary phase are presented in a separate protocol posting (NCT number = NCT00970307).
Start Date18 Aug 2010 |
Sponsor / Collaborator |
Feasibility Study of GlaxoSmithKline Biologicals' GSK2202083A Vaccine in Healthy Infants at 2, 4 and 12 Months of Age
This study will evaluate the safety and immunogenicity of GSK Biologicals' GSK2202083 vaccine co-administered with Prevenar 13® at 2, 4 and 12 months of age and with Rotarix™ at 2 and 4 months of age.
Start Date17 May 2010 |
Sponsor / Collaborator |
Immunogenicity and Safety Study of GlaxoSmithKline Biologicals' GSK2202083A Vaccine in Healthy Infants at 2, 3 and 4 Months of Age
This study will evaluate the safety and immunogenicity of GSK Biologicals' GSK2202083A vaccine co-administered with GSK Biologicals' 10-valent pneumococcal conjugate (GSK1024850A) vaccine given as a three-dose primary vaccination course at 2, 3 and 4 months of age.
Start Date13 Aug 2009 |
Sponsor / Collaborator |
100 Clinical Results associated with Heptavalent combination vaccine(GlaxoSmithKline Plc)
Login to view more data
100 Translational Medicine associated with Heptavalent combination vaccine(GlaxoSmithKline Plc)
Login to view more data
100 Patents (Medical) associated with Heptavalent combination vaccine(GlaxoSmithKline Plc)
Login to view more data
463
Literatures (Medical) associated with Heptavalent combination vaccine(GlaxoSmithKline Plc)31 Dec 2024·Human Vaccines & Immunotherapeutics
Production of monoclonal antibodies against botulinum neurotoxin in
Nicotiana benthamiana
Article
Author: Bulaon, Christine Joy I ; Sangprasat, Kornchanok ; Jirarojwattana, Perawat ; Wongwatanasin, Apidsada ; Rattanapisit, Kaewta ; Srisangsung, Theerakarn ; Phoolcharoen, Waranyoo
01 Feb 2023·Archives of Virology
Assessment of the potency and effectiveness of a heptavalent oil-adjuvanted (ISA 206) foot-and-mouth disease vaccine in Egypt
Article
Author: Badr, Yassien ; Wasfy, Momtaz ; Amer, Haitham M ; Nayel, Mohamed ; Magouz, Asmaa ; El-Sayed, Magdy M ; Maklad, Nada ; Attia, Mohamed ; Abdelmegeid, Mohamed ; Bazid, Abdel-Hamid
01 Jul 2019·Revista Argentina de MicrobiologíaQ4 · BIOLOGY
Clinical and microbiological characteristics of community-acquired pneumonia associated with Streptococcus pneumoniae in adult patients in Mexico
Q4 · BIOLOGY
ArticleOA
Author: Guajardo-Lara, Claudia Elena ; Camacho-Ortiz, Adrián ; Echaniz-Aviles, Gabriela ; Román-Mancha, Alma Lucía ; Ayala-Gaytán, Juan Jacobo ; Morfín-Otero, Rayo ; Garza-González, Elvira ; Soto-Nogueron, Araceli ; Rodríguez-Noriega, Eduardo ; Carnalla-Barajas, Maria Noemí
5
News (Medical) associated with Heptavalent combination vaccine(GlaxoSmithKline Plc)06 Aug 2024
Second Quarter 2024 Total Revenues of $254.7 million, above the prior guidance rangeSecond Quarter 2024 Net Loss of $283.1 million and Adjusted EBITDA of $(10.1) millionUpdates FY 2024 guidance
GAITHERSBURG, Md., Aug. 06, 2024 (GLOBE NEWSWIRE) -- Emergent BioSolutions Inc. (NYSE: EBS) today reported financial results for the second quarter ended June 30, 2024.
"In the first half of the year, we made great progress to stabilize our financial position by strategically divesting assets, resolving several legacy issues, and securing operational cash flow and working capital improvements," said Joe Papa, president and CEO at Emergent. “As a result, we expect to exceed $200 million in debt reduction by the end of the year. With a sharpened focus on our core products, operationalizing a leaner, more flexible footprint with our customers and patients at the center of our efforts, we are well positioned to enhance Emergent’s leadership position in public health preparedness."
FINANCIAL HIGHLIGHTS(1)
Q2 2024 vs. Q2 2023
($ in millions, except per share amounts)Q2 2024Q2 2023% Change Total Revenues$254.7 $337.9 (25)%Net Loss$(283.1)$(261.4)(8)%Net Loss per Diluted Share$(5.38)$(5.16)(4)%Adjusted Net Loss(2)$(122.0)$(53.3)(129)%Adjusted Net Loss per Diluted Share(2)$(2.32)$(1.05)(121)%Adjusted EBITDA(2)$(10.1)$55.9 (118)%Total Segment Gross Margin %(2)(19)% 42% Total Segment Adjusted Gross Margin %(2) 26% 43%
Year to Date ("YTD") 2024 vs. YTD 2023
($ in millions, except per share amounts)YTD 2024YTD 2023% ChangeTotal Revenues$555.1 $502.2 11 %Net Loss$(274.1)$(447.6)39 %Net Loss per Diluted Share$(5.23)$(8.86)41 %Adjusted Net Loss(2)$(90.9)$(216.8)58 %Adjusted Net Loss per Diluted Share(2)$(1.73)$(4.29)60 %Adjusted EBITDA(2)$56.8 $(45.6)*Total Segment Gross Margin %(2) 18 % 30 % Total Segment Adjusted Gross Margin %(2) 39 % 31 %
* % change is greater than +/- 200%
SELECT Q2 2024 AND OTHER RECENT BUSINESS UPDATES
Secured $250 million in U.S. government contract award modifications for four medical countermeasuresAnnounced a $30 million definitive agreement to sell the Baltimore-Camden manufacturing site, which is expected to close in the third quarter of 2024, subject to the satisfaction or waiver of customary closing conditionsReceived $50 million in the third quarter related to the resolution of the contract dispute with Janssen Pharmaceuticals, Inc.Received $7 million for sale of an unimproved, empty building in Canton, MassachusettsExpected receipt of $10 million development milestone payment in the third quarter of 2024 from Bavarian Nordic as part of the sale of the Travel Health BusinessReceived $75 million for the sale of our RSDL® (Reactive Skin Decontamination Lotion) product to SERB Pharmaceuticals, subject to customary adjustments based on inventory value at closing
SECOND QUARTER 2024 FINANCIAL PERFORMANCE(1)
Revenues
The Company uses the following categories in discussing product/service level revenues:
NARCAN® — comprises contributions from NARCAN® Nasal SprayOther Commercial Products - comprised former contributions from Vaxchora® and Vivotif®, which we sold to Bavarian Nordic as part of our travel health business in May 2023Anthrax MCM — comprises contributions from CYFENDUS®, previously known as AV7909, BioThrax®, Anthrasil® and RaxibacumabSmallpox MCM — comprises contributions from ACAM2000®, VIGIV and TEMBEXA®Other Products — comprises contributions from BAT® and RSDL®Bioservices — comprises service and lease revenues from the Bioservices business
($ in millions)Q2 2024Q2 2023% ChangeProduct sales, net:(3) NARCAN®$120.0 $133.9 (10 )%Other Commercial Products — 4.0 NMAnthrax MCM 38.7 21.1 83 %Smallpox MCM 17.9 123.8 (86 )%Other Products 6.8 19.4 (65 )%Total Product sales, net$183.4 $302.2 (39 )% Bioservices: Services$64.5 $26.4 144 %Leases 0.2 2.7 (93 )%Total Bioservices revenues$64.7 $29.1 122 % Contracts and grants$6.6 $6.6 — % Total revenues$254.7 $337.9 (25 )% NM - Not Meaningful
Products Sales, net
NARCAN®
For Q2 2024, revenues from NARCAN® (naloxone HCl) Nasal Spray decreased $13.9 million, or 10%, as compared with Q2 2023. The decrease was primarily driven by an unfavorable price and volume mix in 2024 to U.S. public interest channels and lower Canadian market sales, partially offset by higher sales of over-the-counter (“OTC”) NARCAN® through wholesaler channels, which launched in the third quarter of 2023.
Other Commercial Products
For Q2 2024, we did not receive revenues from Other Commercial Products, representing a decrease of $4.0 million compared with Q2 2023. During the second quarter of 2023, the Company sold Vivotif® and Vaxchora® to Bavarian Nordic as part of our travel health business.
Anthrax MCM
For Q2 2024, revenues from Anthrax MCM increased $17.6 million, or 83%, as compared with Q2 2023. The increase reflects the impact of timing of sales related to CYFENDUS® and BioThrax®, partially offset by a decrease in Anthrasil® sales, due to timing. Anthrax vaccine product sales are primarily made under annual purchase options exercised by the USG. Fluctuations in revenues result from the timing of the exercise of annual purchase options, the timing of USG purchases, the availability of governmental funding and the Company's delivery of orders that follow.
Smallpox MCM
For Q2 2024, revenues from Smallpox MCM decreased $105.9 million, or 86%, as compared with Q2 2023. The decrease was primarily due to timing of USG purchases of ACAM2000® and VIGIV. Fluctuations in revenues from Smallpox MCM result from the timing of the exercise of annual purchase options in the existing procurement contracts, the timing of USG purchases, the availability of governmental funding and the Company's delivery of orders that follow.
Other Products
For Q2 2024, revenues from Other Product sales decreased $12.6 million, or 65%, as compared with Q2 2023. The decrease was due to lower BAT® and RSDL® product sales, due to timing of deliveries.
Bioservices Revenues
Services
For Q2 2024, revenues from Bioservices services increased $38.1 million, or 144%, as compared with Q2 2023. The increase was primarily attributable to the $50.0 million arbitration settlement (the "Settlement Agreement") with Janssen Pharmaceuticals, Inc. (“Janssen”), one of the Janssen Pharmaceutical Companies of Johnson & Johnson, related to the 2022 termination of the manufacturing services agreement with Janssen (the “Janssen Agreement”), coupled with an increase in production at our Camden facility. The increase was partially offset by lower production at the Company's Canton and Winnipeg facilities coupled with the prior year quarter recognition of revenue related to the resolution of a customer’s outstanding obligation.
Leases
For Q2 2024, revenues from Bioservices leases decreased $2.5 million, or 93%, as compared with Q2 2023. The decrease was related to the completion of a lease for a Bioservices customer at our Canton facility.
Contracts and Grants
For Q2 2024, revenues from contracts and grants was consistent with Q2 2023.
Operating Expenses
($ in millions)Q2 2024Q2 2023% ChangeCost of Commercial product sales$53.4 $54.4 (2 )%Cost of MCM product sales 31.1 80.5 (61 )%Cost of Bioservices 211.6 55.7 *Impairment of long-lived assets 27.2 306.7 (91 )%Research and development (“R&D”) 32.7 26.0 26 %Selling, general and administrative (“SG&A”) 85.9 91.4 (6 )%Amortization of intangible assets 16.3 16.1 1 %Total operating expenses$458.2 $630.8 (27 )% * % change is greater than +/- 200%
Cost of Commercial Product Sales
For Q2 2024, cost of Commercial Product sales decreased $1.0 million, or 2%, as compared with Q2 2023. The decrease was primarily due to no current period costs related to Vivotif® and Vaxchora®, which were sold to Bavarian Nordic as part of our travel health business, partially offset by higher NARCAN® expense as a result of increased unit volume.
Cost of MCM Product Sales
For Q2 2024, cost of MCM Product sales decreased $49.4 million, or 61%, as compared with Q2 2023. The decrease was primarily due to lower sales of ACAM2000®, BAT®, RSDL® and Anthrasil®, lower allocations to Cost of MCM Product sales at our Bayview facility and a reduction in Trobigard® related costs, due to the Belgium Federal Agency for Medicines and Health Products' approval of the Company's request to revoke the Market Authorization for Trobigard® (the "Trobigard® revocation"). This decrease was partially offset by higher shutdown costs, Raxibacumab inventory reserves and overhead allocations at our Winnipeg facility.
Cost of Bioservices
For Q2 2024, cost of Bioservices increased $155.9 million as compared with Q2 2023. The increase was primarily due to the Settlement Agreement with Janssen and resulting write-down of related assets to net realizable value, partially offset by a decrease in production at the Company's Canton facility and a decrease in overhead costs at our Maryland facilities.
Impairment of Long-Lived Assets
For Q2 2024, Impairment of long-lived assets decreased $279.5 million, or 91%, as compared with Q2 2023. The decrease was due to a $27.2 million non-cash impairment charge in the second quarter of 2024 related to our Bayview and Rockville asset groups within the Bioservices reporting unit, compared to a $306.7 million non-cash impairment charge recorded in the second quarter of 2023 related to our Camden, Bayview and Rockville asset groups within the Bioservices reporting unit.
Research and Development Expenses
For Q2 2024, R&D expenses increased $6.7 million, or 26%, as compared with Q2 2023. The increase was primarily due to write-offs related to program terminations during the period and an increase in R&D overhead and severance costs. The increase was partially offset by the sale of our development program for CHIKV VLP to Bavarian Nordic and reduction in related overhead costs driven by the headcount reductions and an overall decrease in spend for funded projects.
Selling, General and Administrative Expenses
For Q2 2024, SG&A expenses decreased $5.5 million, or 6%, as compared with Q2 2023. The decrease was primarily due to lower employee related expenses and compensation as a result of restructuring initiatives during 2023, coupled with a decrease in marketing expense. The decrease was partially offset by higher legal services fees for disputes and other corporate initiatives, as well as higher restructuring costs.
ADDITIONAL FINANCIAL INFORMATION(1)
Capital Expenditures
($ in millions)Q2 2024Q2 2023% Change Capital expenditures$4.6 $12.5 (63 )%Capital expenditures as a % of total revenues 2 % 4 %
For Q2 2024, capital expenditures decreased largely due to lower product development activities across the Company’s facilities.
SEGMENT INFORMATION
In the fourth quarter of 2023, we realigned our reportable operating segments to reflect recent changes in our internal operating and reporting process. The Company now manages the business with a focus on three reportable segments: (1) the Commercial Products segment consisting of our NARCAN® and other commercial products that were sold as part of our travel health business in the second quarter of 2023; (2) the MCM Products segment consisting of the Anthrax - MCM, Smallpox - MCM and Other products and (3) the services segment (“Services”) consisting of our Bioservices. The Company evaluates the performance of these reportable segments based on revenue and segment adjusted gross margin, which is a non-GAAP financial measure. Segment revenue includes external customer sales, but does not include inter-segment services. The Company does not allocate contracts and grants, R&D, SG&A, amortization of intangible assets, interest and other income (expense) or taxes to its evaluation of the performance of these segments.
SECOND QUARTER 2024 SEGMENT RESULTS
($ in millions)Commercial Products Quarter Ended June 30, 2024 2023 $ Change% ChangeRevenues$120.0 $137.9 $(17.9)(13 )%Cost of sales 53.4 54.4 (1.0)(2 )% Gross margin**$66.6 $83.5 $(16.9)(20 )% Gross margin %** 56% 61%
Segment adjusted gross margin(2)$66.6 $83.5 $(16.9)(20 )% Segment adjusted gross margin %(2) 56% 61%
** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues.
Commercial Products gross margin decreased $16.9 million, or 20%, to $66.6 million in the quarter, as compared with $83.5 million in the prior year quarter. Commercial Products gross margin percentage decreased 5 percentage points to 56% for the quarter ended June 30, 2024. The decrease was largely due to an unfavorable price and volume mix in 2024 for NARCAN® products, partially offset by the sale of the products associated with our travel health business to Bavarian Nordic. Commercial Products segment adjusted gross margin is consistent with gross margin.
($ in millions)MCM Products Quarter Ended June 30, 2024 2023 $ Change% ChangeRevenues$63.4 $164.3 $(100.9)(61 )%Cost of sales 31.1 80.5 (49.4)(61 )% Gross margin**$32.3 $83.8 $(51.5)(61 )% Gross margin %** 51 % 51 % Add back:
Changes in fair value of contingent consideration$0.1 $0.4 $(0.3)(75 )% Restructuring costs 2.7 — 2.7 NMInventory step-up provision — 1.9 (1.9) NMSegment adjusted gross margin(2)$35.1 $86.1 $(51.0)(59 )% Segment adjusted gross margin %(2) 55 % 52 %
** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues. NM - Not Meaningful
MCM Products gross margin decreased $51.5 million, or 61%, to $32.3 million in the quarter, as compared with $83.8 million in the prior year quarter. MCM Products gross margin percentage was consistent at 51% for the quarter ended June 30, 2024. MCM Product segment adjusted gross margin in the current year period excludes the impact of non-cash items related to the impact of restructuring costs of $2.7 million and the changes in the fair value of contingent consideration of $0.1 million.
($ in millions)ServicesQuarter Ended June 30, 2024 2023 $ Change% ChangeRevenues$64.7 $29.1 $35.6 122 %Cost of services 211.6 55.7 155.9 *Gross margin**$(146.9)$(26.6)$(120.3) *Gross margin %**(227 )%(91)% Add back: Settlement charge, net$110.2 $— 110.2 NMRestructuring costs 0.4 — 0.4 NMSegment adjusted gross margin(2)$(36.3)$(26.6)$(9.7)(36 )%Segment adjusted gross margin %(2)(56 )%(91)%
* % change is greater than +/- 200% ** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues.NM - Not Meaningful
Services gross margin decreased $120.3 million to $(146.9) million in the quarter, as compared with $(26.6) million in the prior year quarter. Services gross margin percentage decreased 136 percentage points to (227)% for the quarter ended June 30, 2024. The decrease was primarily due to the Settlement Agreement with Janssen and resulting revenue and write-down of related assets, coupled with lower production at the Company's Canton and Winnipeg facilities, partially offset by an increase in production at the Company’s Camden facility and a decrease in overhead costs at our Maryland facilities. Services segment adjusted gross margin in the current year period excludes the impact of the settlement charge, net of $110.2 million and restructuring costs of $0.4 million.
YTD 2024 SEGMENT RESULTS
($ in millions)Commercial ProductsSix Months Ended June 30, 2024 2023 $ Change% ChangeRevenues$238.5 $244.1 $(5.6)(2 )%Cost of sales 105.5 100.2 5.3 5 %Gross margin**$133.0 $143.9 $(10.9)(8 )%Gross margin %** 56 % 59 %
Segment adjusted gross margin(2)$133.0 $143.9 $(10.9)(8 )%Segment adjusted gross margin %(2) 56 % 59 %
** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues.
Commercial Products gross margin decreased $10.9 million, or 8%, to $133.0 million for the six months ended June 30, 2024, as compared with $143.9 million for the six months ended June 30, 2023. Commercial Products gross margin percentage decreased 3 percentage points to 56% in 2024. The decrease was largely due to an unfavorable price and volume mix in 2024 for NARCAN® products, partially offset by the sale of the products associated with our travel health business to Bavarian Nordic. Commercial Products segment adjusted gross margin is consistent with gross margin.
($ in millions)MCM ProductsSix Months Ended June 30, 2024 2023 $ Change% ChangeRevenues$218.8 $201.5 $17.3 9 %Cost of sales 93.3 135.9 (42.6)(31 )%Gross margin**$125.5 $65.6 $59.9 91 %Gross margin %** 57 % 33 % Add back: Changes in fair value of contingent consideration$0.6 $0.7 $(0.1)(14 )%Inventory step-up provision — 1.9 (1.9) NM Restructuring costs 2.6 2.0 0.6 30 %Segment adjusted gross margin(2)$128.7 $70.2 $58.5 83 %Segment adjusted gross margin %(2) 59 % 35 %
** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues.NM - Not Meaningful
MCM Products gross margin increased $59.9 million, or 91%, to $125.5 million for the six months ended June 30, 2024, as compared with $65.6 million for the six months ended June 30, 2023. MCM Products gross margin percentage increased 24 percentage points to 57% for the six months ended June 30, 2024. The increase was largely due to overall higher sales volumes with a favorable product mix that was weighted more heavily to higher margin products coupled with lower allocations to Cost of MCM Product sales at our Bayview facility and lower shutdown related costs, a reduction in Trobigard® related costs, due to the Trobigard® revocation, and realization of previously adjusted inventory values. MCM Product segment adjusted gross margin excludes the impact of restructuring costs of $2.6 million and changes in the fair value of contingent consideration of $0.6 million.
($ in millions)ServicesSix Months Ended June 30,20242023$ Change% ChangeRevenues$83.2 $43.5 $39.7 91%Cost of services 241.9 107.4 134.5 125 %Gross margin**$(158.7)$(63.9)$(94.8)(148)%Gross margin %**(191)%(147)% Add back: Settlement charge, net$110.2 $— $110.2 NMRestructuring costs 0.2 — 0.2 NMSegment adjusted gross margin(2)$(48.3)$(63.9)$15.6 24 %Segment adjusted gross margin %(2)(58)%(147)%
** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues.NM - Not Meaningful
Services gross margin decreased $94.8 million, or 148%, to ($158.7) million for the six months ended June 30, 2024, as compared with ($63.9) million for the six months ended June 30, 2023. Services gross margin percentage decreased 44 percentage points to (191)% for the six months ended June 30, 2024. The decrease was primarily due to the Settlement Agreement with Janssen and resulting revenue and write-down of related assets, partially offset by higher production at the Company’s Camden Facility. Services segment adjusted gross margin in the current year period excludes the impact of settlement charge, net of $110.2 million and restructuring costs of $0.2 million.
2024 FINANCIAL FORECAST
The Company provides the following updated financial forecast for full year 2024 and initial forecast for Q3 2024, in both instances reflecting management's expectations based on the most current information available.
Full Year 2024
METRIC($ in millions)
Updated Range(as of 08/06/2024)Previous Range(as of 05/01/2024)Previous Range(as of 03/06/2024)Total revenues$1,050 - $1,125$1,000 - $1,100$900 - $1,100Net loss$(314) - $(274)$(148) - $(98)$(183) - $(133)Adjusted net loss(2)$(115) - $(75)$(65) - $(15)$(130) - $(80)Adjusted EBITDA(2)$140 - $180$125 - $175$50 - $100Total segment adjusted gross margin %(2)42% - 45%44% - 47%40% - 45% Segment Level Revenue(4) Commercial Products$450 - $480$460 - $500$460 - $500MCM Products$455 - $490$440 - $490$340 - $490Services(5)$120 - $130$70 - $80$70 - $80
Key Assumptions($ and shares in millions)Updated Range(as of 08/06/2024)Interest expense~$82R&D~7% of RevenueWeighted avg. fully diluted share count~53Capex~$30Depreciation & amortization~$111
Q3 2024
METRIC($ in millions)
Q3 2024 ForecastTotal revenues$265 - $315
FOOTNOTES
(1) All financial information included in this release is unaudited.
(2) See “Non-GAAP Financial Measures” and the "Reconciliation of Non-GAAP Financial Measures" tables for the definitions and reconciliations of these non-GAAP financial measures to the most closely related GAAP financial measures.
(3) Product sales, net are reported net of variable consideration including returns, rebates, wholesaler fees and prompt pay discounts in accordance with U.S. generally accepted accounting principles.
(4) Our Commercial Products forecast consists solely of NARCAN® Nasal Spray, as our Other Commercial Products, including Vivotif® and Vaxchora®, were sold to Bavarian Nordic as part of our travel health business in May 2023.
(5) Our Services revenue forecast includes $50.0 million related to the Settlement Agreement with Janssen.
CONFERENCE CALL, PRESENTATION SUPPLEMENT AND WEBCAST INFORMATION
Company management will host a conference call at 5:00 pm eastern time today, August 6, 2024, to discuss these financial results. The conference call and presentation supplement can be accessed from the Company's website or through the following:
By phone Advance registration is required. Visit https://register.vevent.com/register/BI59bc8013f2c54ef296711d52848b0d4d to register and receive an email with the dial-in number, passcode and registrant ID.By webcast Visit https://edge.media-server.com/mmc/p/p7f4ooy9 A replay of the call can be accessed from the Emergent website.
ABOUT EMERGENT BIOSOLUTIONS INC.
At Emergent, our mission is to protect and enhance life. We develop, manufacture, and deliver protections against public health threats through a pipeline of innovative vaccines and therapeutics. For over 25 years, we have been at work defending people from things we hope will never happen—so that we are prepared just in case they ever do. We do what we do because we see the opportunity to create a better, more secure world. One where preparedness empowers protection from the threats we face. And peace of mind prevails. In working together, we envision protecting or enhancing 1 billion lives by 2030. For more information, visit our website and follow us on LinkedIn, Twitter, and Instagram.
NON-GAAP FINANCIAL MEASURES
In the accompanying analysis of financial information, we sometimes use information derived from consolidated and segment financial information that may not be presented in our financial statements or prepared in accordance with generally accepted accounting principles in the United States (“GAAP”). Certain of these financial measures are considered not in conformity with GAAP (“non-GAAP financial measures”) under the United States Securities and Exchange Commission (“SEC”) rules. Specifically, we have referred to the following non-GAAP financial measures:
Adjusted Net LossAdjusted Net Loss per Diluted ShareAdjusted EBITDATotal Segment RevenuesTotal Segment Gross MarginTotal Segment Gross Margin %Total Segment Adjusted Gross MarginTotal Segment Adjusted Gross Margin %Segment Adjusted Gross MarginSegment Adjusted Gross Margin %
We define Adjusted Net Loss and Adjusted Net Loss per Diluted Share, which are non-GAAP financial measures, as net loss and net loss per diluted share, respectively, excluding the impact of changes in fair value of contingent consideration, acquisition and divestiture-related costs, severance and restructuring costs, other income (expense) items, settlement charge, net, exit and disposal costs, impairment charges, gain (loss) on sale of business and assets held for sale and non-cash amortization charges. We use Adjusted Net Loss for the purpose of calculating Adjusted Net Loss per Diluted Share. Management uses Adjusted Net Loss per Diluted Share to assess total Company operating performance on a consistent basis. We believe that these non-GAAP financial measures, when considered together with our GAAP financial results and GAAP financial measures, provide management and investors with an additional understanding of our business operating results, including underlying trends.
We define Adjusted EBITDA, which is a non-GAAP financial measure, as consolidated net income (loss) before income tax provision (benefit), interest expense, net, depreciation, amortization of intangible assets, changes in fair value of contingent consideration, severance and restructuring costs, other income (expense) items, settlement charge, net, impairments, gain (loss) on sale of business and assets held for sale and acquisition and divestiture-related costs. We believe that this non-GAAP financial measure, when considered together with our GAAP financial results and GAAP financial measures, provides management and investors with a more complete understanding of our operating results, including underlying trends. In addition, EBITDA is a common alternative measure of operating performance used by many of our competitors. It is used by investors, financial analysts, rating agencies and others to value and compare the financial performance of companies in our industry, although it may be defined differently by different companies. Therefore, we also believe that this non-GAAP financial measure, considered along with corresponding GAAP financial measures, provides management and investors with additional information for comparison of our operating results with the operating results of other companies.
We have included the definitions of Segment Gross Margin and Segment Gross Margin %, which are GAAP financial measures, below in order to more fully define the components of certain non-GAAP financial measures presented in this press release. We define Segment Gross Margin, as a segment's revenues, less a segment's cost of sales or services. We define Segment Gross Margin %, as Segment Gross Margin as a percentage of a segments revenues. We define Segment Adjusted Gross Margin, which is a non-GAAP financial measure as Segment Gross Margin excluding the impact of restructuring costs and non-cash items related to changes in the fair value of contingent consideration, settlement charge, net and inventory step-up provision. We define Segment Adjusted Gross Margin %, which is a non-GAAP financial measure, as Segment Adjusted Gross Margin as a percentage of a segment's revenues.
We define Total Segment Revenues, which is a non-GAAP financial measure, as our Total Revenues, less contracts and grants revenue, which is also equal to the sum of the revenues of our reportable operating segments. We define Total Segment Gross Margin, which is a non-GAAP financial measure, as Total Segment Revenues less our aggregate cost of sales or services. We define Total Segment Gross Margin %, which is a non-GAAP financial measure, as Total Segment Gross Margin as a percentage of Total Segment Revenues. We define Total Segment Adjusted Gross Margin, which is a non-GAAP financial measure, as Total Segment Gross Margin, excluding the impact of restructuring costs and changes in the fair value of contingent consideration. We define Total Segment Adjusted Gross Margin %, which is a non-GAAP financial measure, as Total Segment Adjusted Gross Margin as a percentage of Total Segment Revenues.
Non-GAAP financial measures are not defined in the same manner by all companies and may not be comparable with other similarly titled measures of other companies. The determination of the amounts that are excluded from these non-GAAP financial measures are a matter of management judgment and depend upon, among other factors, the nature of the underlying expense or income amounts. Non-GAAP financial measures should be considered in addition to, but not as a substitute for or superior to, the information contained in our Consolidated Statements of Operations and Consolidated Statements of Cash Flows. Reconciliations of these non-GAAP financial measures to the most directly comparable GAAP financial measures are included in the financial tables accompanying this press release.
SAFE HARBOR STATEMENT
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical fact, including statements regarding the future performance of the Company or any of our businesses, our business strategy, future operations, future financial position, future revenues and earnings, our ability to achieve the objectives of our restructuring initiatives and divestitures, including our future results, projected costs, prospects, plans and objectives of management, are forward-looking statements. We generally identify forward-looking statements by using words like “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “forecast,” “future,” “goal,” “intend,” “may,” “plan,” “position,” “possible,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would,” and similar expressions or variations thereof, or the negative thereof, but these terms are not the exclusive means of identifying such statements. Forward-looking statements are based on our current intentions, beliefs, assumptions and expectations regarding future events based on information that is currently available. Readers should realize that if underlying assumptions prove inaccurate or if known or unknown risks or uncertainties materialize, actual results could differ materially from our expectations. Readers are, therefore, cautioned not to place undue reliance on any forward-looking statement contained herein. Any such forward-looking statement speaks only as of the date of this press release, and, except as required by law, we do not undertake any obligation to update any forward-looking statement to reflect new information, events or circumstances.
There are a number of important factors that could cause our actual results to differ materially from those indicated by such forward-looking statements, including, among others, the availability of USG funding for contracts related to procurement of our medical countermeasure ("MCM") products, including CYFENDUS® (Anthrax Vaccine Adsorbed (AVA), Adjuvanted), previously known as AV7909, BioThrax® (Anthrax Vaccine Adsorbed), and ACAM2000®, (Smallpox (Vaccinia) Vaccine, Live), among others, as well as contracts related to development of medical countermeasures; the availability of government funding for our other commercialized products, including EbangaTM (ansuvimab-zykl) and BAT® (Botulism Antitoxin Heptavalent (A,B,C,D,E,F,G)-(Equine)); our ability to meet our commitments to quality and compliance in all of our manufacturing operations; our ability to negotiate additional USG procurement or follow-on contracts for our MCM products that have expired or will be expiring; the commercial availability and acceptance of over-the-counter NARCAN® (naloxone HCl) Nasal Spray; the impact of a generic and competitive marketplace on NARCAN® Nasal Spray and future NARCAN® Nasal Spray sales; our ability to perform under our contracts with the USG, including the timing of and specifications relating to deliveries; our ability to provide Bioservices (as defined below) for the development and/or manufacture of product and/or product candidates of our customers at required levels and on required timelines; the ability of our contractors and suppliers to maintain compliance with current good manufacturing practices and other regulatory obligations; our ability to negotiate further commitments related to the collaboration and deployment of capacity toward future commercial manufacturing under our existing Bioservices contracts; our ability to collect reimbursement for raw materials and payment of services fees from our Bioservices customers; the results of pending stockholder litigation and government investigations and their potential impact on our business; our ability to comply with the operating and financial covenants and the capital raise requirements by the stated deadline required by our revolving credit facility and our term loan facility under a senior secured credit agreement, dated October 15, 2018, between the Company and multiple lending institutions, as amended from time to time, as well as our 3.875% Senior Unsecured Notes due 2028; our ability to maintain adequate internal control over financial reporting and to prepare accurate financial statements in a timely manner; our ability to resolve the going concern qualification in our consolidated financial statements and otherwise successfully manage our liquidity in order to continue as a going concern; the procurement of our product candidates by USG entities under regulatory authorities that permit government procurement of certain medical products prior to FDA marketing authorization, and corresponding procurement by government entities outside of the United States; our ability to realize the expected benefits of the sale of our travel health business to Bavarian Nordic, the sale of RSDL® to SERB Pharmaceuticals and the pending sale of our drug product facility in Baltimore-Camden to Bora Pharmaceuticals Injectibles Inc.; the impact of the organizational changes we announced in January 2023, August 2023 and May 2024; our ability to identify and acquire companies, businesses, products or product candidates that satisfy our selection criteria; the impact of cyber security incidents, including the risks from the unauthorized access, interruption, failure or compromise of our information systems or those of our business partners, collaborators or other third parties; the success of our commercialization, marketing and manufacturing capabilities and strategy; and the accuracy of our estimates regarding future revenues, expenses, capital requirements and needs for additional financing. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from our expectations in any forward-looking statement. Readers should consider this cautionary statement, as well as the risks identified in our periodic reports filed with the Securities and Exchange Commission, when evaluating our forward-looking statements.
Trademarks
Emergent®, BioThrax®, BaciThrax®, BAT®, Trobigard®, Anthrasil®, CNJ-016®, ACAM2000®, NARCAN®, CYFENDUS®, TEMBEXA® and any and all Emergent BioSolutions Inc. brands, products, services and feature names, logos and slogans are trademarks or registered trademarks of Emergent BioSolutions Inc. or its subsidiaries in the United States or other countries. All other brands, products, services and feature names or trademarks are the property of their respective owners, including RSDL® (Reactive Skin Decontamination Lotion), which was acquired by SERB on July 31, 2024.
Investor ContactRich LindahlExecutive Vice President, Chief Financial Officerlindahlr@ebsi.comMedia ContactAssal HellmerVice President, Communicationsmediarelations@ebsi.com
Emergent BioSolutions Inc. Consolidated Balance Sheets(unaudited, in millions, except per share data) June 30, December 31, 2024 2023 ASSETS Current assets: Cash and cash equivalents$69.7 $111.7 Restricted cash 1.3 — Accounts receivable, net 196.3 191.0 Inventories, net 317.5 328.9 Prepaid expenses and other current assets 36.0 47.9 Assets held for sale 33.7 — Total current assets 654.5 679.5
Property, plant and equipment, net 306.2 382.8 Intangible assets, net 534.1 566.6 Other assets 18.7 194.3 Total assets$1,513.5 $1,823.2
LIABILITIES AND STOCKHOLDERS’ EQUITY Current liabilities: Accounts payable$95.9 $112.2 Accrued expenses 17.2 18.6 Accrued compensation 66.7 74.1 Debt, current portion 415.2 413.7 Other current liabilities 14.6 32.7 Liabilities held for sale 10.1 — Total current liabilities 619.7 651.3
Debt, net of current portion 447.0 446.5 Deferred tax liability 34.8 47.2 Other liabilities 25.7 28.9 Total liabilities$1,127.2 $1,173.9
Stockholders’ equity: Preferred stock, $0.001 par value per share; 15.0 shares authorized, no shares issued and outstanding — — Common stock, $0.001 par value per share; 200.0 shares authorized, 58.5 and 57.8 shares issued; 52.9 and 52.2 shares outstanding, respectively. 0.1 0.1 Treasury stock, at cost, 5.6 and 5.6 common shares, respectively (227.7) (227.7)Additional paid-in capital 915.3 904.4 Accumulated other comprehensive loss, net (5.5) (5.7)Accumulated deficit (295.9) (21.8)Total stockholders’ equity$386.3 $649.3 Total liabilities and stockholders’ equity$1,513.5 $1,823.2
Emergent BioSolutions Inc. Consolidated Statements of Operations(unaudited, in millions, except per share data) Three Months Ended June 30, Six Months Ended June 30, 2024 2023 2024 2023 Revenues:
Commercial Product sales$120.0 $137.9 $238.5 $244.1 MCM Product sales 63.4 164.3 218.8 201.5 Total Product sales, net 183.4 302.2 457.3 445.6 Bioservices:
Services 64.5 26.4 82.8 39.0 Leases 0.2 2.7 0.4 4.5 Total Bioservices revenues 64.7 29.1 83.2 43.5 Contracts and grants 6.6 6.6 14.6 13.1 Total revenues 254.7 337.9 555.1 502.2
Operating expenses:
Cost of Commercial Product sales 53.4 54.4 105.5 100.2 Cost of MCM Product sales 31.1 80.5 93.3 135.9 Cost of Bioservices 211.6 55.7 241.9 107.4 Impairment of long-lived assets 27.2 306.7 27.2 306.7 Research and development 32.7 26.0 47.8 66.7 Selling, general and administrative 85.9 91.4 170.6 192.7 Amortization of intangible assets 16.3 16.1 32.5 33.1 Total operating expenses 458.2 630.8 718.8 942.7
Loss from operations (203.5) (292.9) (163.7) (440.5)
Other income (expense):
Interest expense (23.6) (28.6) (47.9) (46.5)Gain (loss) on sale of business and assets held for sale (40.0) 74.9 (40.0) 74.9 Other, net (2.7) (3.6) (6.1) 1.3 Total other income (expense), net (66.3) 42.7 (94.0) 29.7
Loss before income taxes (269.8) (250.2) (257.7) (410.8)Income tax provision 13.3 11.2 16.4 36.8 Net loss$(283.1) $(261.4) $(274.1) $(447.6)
Net loss per common share
Basic$(5.38) $(5.16) $(5.23) $(8.86)Diluted$(5.38) $(5.16) $(5.23) $(8.86)
Shares used in computing net loss per common share
Basic 52.6 50.7 52.4 50.5 Diluted 52.6 50.7 52.4 50.5
Emergent BioSolutions Inc.Consolidated Statements of Cash Flowsunaudited, in millions) Six Months Ended June 30, 2024 2023 Operating Activities Net loss$(274.1) $(447.6)Adjustments to reconcile net loss to net cash used in operating activities: Share-based compensation expense 11.4 15.1 Depreciation and amortization 56.4 67.5 Change in fair value of contingent obligations, net 0.6 0.7 Amortization of deferred financing costs 11.9 9.9 Deferred income taxes (12.4) (6.4)Loss (gain) on sale of business and assets held for sale 40.0 (74.9)Impairment of long-lived assets 27.2 306.7 Other 15.5 9.5 Changes in operating assets and liabilities: Accounts receivable (29.6) (129.8)Inventories (17.5) (24.9)Prepaid expenses and other assets 160.0 (17.8)Accounts payable 0.2 10.9 Accrued expenses and other liabilities 3.8 (13.8)Long-term incentive plan accrual 1.9 2.4 Accrued compensation (7.0) (12.4)Income taxes receivable and payable, net 16.1 14.2 Contract liabilities (19.5) (7.7)Net cash used in operating activities (15.1) (298.4)Investing Activities Purchases of property, plant and equipment (15.4) (27.6)Proceeds from sale of business, net — 270.2 Net cash provided by (used in) investing activities (15.4) 242.6 Financing Activities Proceeds from revolving credit facility 65.0 — Principal payments on revolving credit facility (61.5) (347.8)Principal payments on term loan facility (7.9) (156.8)Proceeds from share-based compensation activity 0.7 1.3 Taxes paid for share-based compensation activity (0.6) (2.3)Debt issuance costs (5.9) — Proceeds from at-the-market sale of stock, net of commissions and expenses — 8.2 Net cash used in financing activities: (10.2) (497.4)Effect of exchange rate changes on cash, cash equivalents and restricted cash — (0.8)Net change in cash, cash equivalents and restricted cash (40.7) (554.0)Cash, cash equivalents and restricted cash, beginning of period 111.7 642.6 Cash, cash equivalents and restricted cash, end of period$71.0 $88.6 Supplemental cash flow disclosures: Cash paid for interest$36.0 $38.8 Cash paid for income taxes$25.9 $26.9 Non-cash investing and financing activities: Purchases of property, plant and equipment unpaid at period end$2.9 $7.7 Gain on extinguishment of debt$0.3 $— Reconciliation of cash and cash equivalents and restricted cash: Cash and cash equivalents$69.7 $88.6 Restricted cash 1.3 — Total$71.0 $88.6
Emergent BioSolutions, Inc.Reconciliation of Non-GAAP Financial MeasuresReconciliation of Net Loss and Net Loss per Diluted Share to Adjusted Net Loss and Adjusted Net Loss per Diluted Share(1) ($ in millions, except per share value)Three Months Ended June 30, Six Months Ended June 30, 2024 2023 2024 2023 SourceNet loss$(283.1)$(261.4) $(274.1)$(447.6) Adjustments:
Non-cash amortization charges$21.1 $25.0 $44.3 $43.0 Amortization of intangible assets, Other IncomeChanges in fair value of contingent consideration 0.1 0.4 0.6 0.7 Cost of MCM ProductsImpairments 27.2 306.7 27.2 306.7 Impairment of long-lived assetsSeverance and restructuring costs 17.1 4.1 16.6 13.8 Cost of MCM Products, Cost of Services, SG&A and R&DInventory step-up provision — 1.9 — 1.9 Cost of MCM ProductsAcquisition and divestiture costs — 1.8 — 2.9 SG&AExit and disposal costs 13.3 6.1 13.3 6.1 R&DLoss (gain) on sale of business and assets held for sale 40.0 (74.9) 40.0 (74.9)Other Income (Expense)Settlement charge, net 110.2 — 110.2 — Cost of ServicesOther income (expense), net item — — 3.1 — Other Income (Expense)Tax effect (67.9) (63.0) (72.1) (69.4) Total adjustments:$161.1 $208.1 $183.2 $230.8 Adjusted net loss$(122.0)$(53.3) $(90.9)$(216.8) Net loss per diluted share$(5.38)$(5.16) $(5.23)$(8.86) Adjustments:
Non-cash amortization charges$0.40 $0.49 $0.86 $0.85 Amortization of intangible assets, Other IncomeChanges in fair value of contingent consideration — 0.01 0.01 0.01 Cost of MCM ProductsImpairments 0.52 6.05 0.52 6.07 Impairment of long-lived assetsSeverance and restructuring costs 0.32 0.08 0.32 0.27 Cost of MCM Products, Cost of Services, SG&A and R&DInventory step-up provision — 0.04 — 0.04 Cost of MCM ProductsAcquisition and divestiture costs — 0.04 — 0.06 SG&AExit and disposal costs 0.25 0.12 0.25 0.12 R&DLoss (gain) on sale of business and assets held for sale 0.76 (1.48) 0.76 (1.48)Other Income (Expense)Settlement charge, net 2.10 — 2.10 — Cost of ServicesOther income (expense), net item — — 0.06 — Other Income (Expense)Tax effect (1.29) (1.24) (1.38) (1.37) Total adjustments:$3.06 $4.11 $3.50 $4.57 Adjusted net loss per diluted share$(2.32)$(1.05) $(1.73)$(4.29) Diluted shares used in computing Adjusted net loss per diluted share 52.6 50.7 52.4 50.5
Emergent BioSolutions, Inc.Reconciliation of Net Loss to Adjusted EBITDA(1) ($ in millions)Three Months Ended June 30, Six Months Ended June 30, 2024 2023 2024 2023 Net loss$(283.1)$(261.4) $(274.1)$(447.6)Adjustments:
Depreciation & amortization$28.5 $32.9 $56.4 $67.5 Income taxes 13.3 11.2 16.4 36.8 Total interest expense, net 23.3 27.1 47.1 40.5 Impairments 27.2 306.7 27.2 306.7 Inventory step-up provision — 1.9 — 1.9 Changes in fair value of contingent consideration 0.1 0.4 0.6 0.7 Severance and restructuring costs 17.1 4.1 16.6 13.8 Exit and disposal costs 13.3 6.1 13.3 6.1 Acquisition and divestiture costs — 1.8 — 2.9 Loss (gain) on sale of business and assets held for sale 40.0 (74.9) 40.0 (74.9)Settlement charge, net 110.2 — 110.2 — Other income (expense), net item — — 3.1 — Total adjustments$273.0 $317.3 $330.9 $402.0 Adjusted EBITDA$(10.1)$55.9 $56.8 $(45.6)
Emergent BioSolutions, Inc.Reconciliations of Total Revenues to Total Segment Revenues and of Segment and Total Segment Gross Margin and Gross Margin % to Segment and Total Segment Adjusted Gross Margin and Adjusted Gross Margin %(1) Three Months Ended June 30, 2024(unaudited, in millions)Commercial ProductsMCM ProductsServices Total Segment Contracts & Grants Total RevenuesRevenues$120.0 $63.4 $64.7 $248.1 $6.6 $254.7
Cost of sales or services 53.4 31.1 211.6 296.1
Gross margin$66.6 $32.3 $(146.9) $(48.0) Gross margin % 56 % 51%(227)% (19)%
Add back:
Changes in fair value of contingent consideration$— $0.1 $— $0.1 Settlement charge, net — — 110.2 110.2 Restructuring costs — 2.7 0.4 3.1
Adjusted gross margin$66.6 $35.1 $(36.3) $65.4 Adjusted gross margin %(1) 56 % 55 %(56)% 26 %
(1) Total Segment results for the three months ended June 30, 2024 includes $50.0 million attributable to the Settlement Agreement with Janssen. The revenue and cost of services is related to raw materials purchased for the Janssen Agreement which Janssen had not reimbursed. Excluding the revenue and cost of services impacts of the Settlement Agreement, Total Segment Adjusted Gross Margin % would have been 7% higher for the three months ended June 30, 2024.
Three Months Ended June 30, 2023(unaudited, in millions)Commercial ProductsMCM ProductsServices Total SegmentContracts & Grants Total RevenuesRevenues$137.9 $164.3 $29.1 $331.3 $6.6 $337.9
Cost of sales or services 54.4 80.5 55.7 190.6
Gross margin$83.5 $83.8 $(26.6) $140.7 Gross margin % 61% 51%(91 )% 42%
Add back:
Changes in fair value of contingent consideration$— $0.4 $— $0.4 Inventory step-up provision — 1.9 — 1.9
Adjusted gross margin$83.5 $86.1 $(26.6) $143.0 Adjusted gross margin % 61% 52%(91 )% 43%
Emergent BioSolutions, Inc.Reconciliations of Total Revenues to Total Segment Revenues and of Segment and Total Segment Gross Margin and Gross Margin % to Segment and Total Segment Adjusted Gross Margin and Adjusted Gross Margin %(1) Six Months Ended June 30, 2024(unaudited, in millions)Commercial ProductsMCM ProductsServices1 Total SegmentContracts & Grants Total RevenuesRevenues$238.5 $218.8 $83.2 $540.5 $14.6 $555.1
Cost of sales or services 105.5 93.3 241.9 440.7
Gross margin$133.0 $125.5 $(158.7) $99.8 Gross margin % 56 % 57 %(191 )% 18 %
Add back:
Changes in fair value of contingent consideration$— $0.6 — $0.6 Settlement charge, net — — $110.2 110.2 Restructuring costs — 2.6 0.2 2.8
Adjusted gross margin$133.0 $128.7 $(48.3) $213.4 Adjusted gross margin %(1) 56 % 59 %(58 )% 39 %
(1) Total Segment results for the six months ended June 30, 2024 includes $50.0 million attributable to the Settlement Agreement with Janssen. The revenue and cost of services is related to raw materials purchased for the Janssen Agreement which Janssen had not reimbursed. Excluding the impacts of the Settlement Agreement, Total Segment Adjusted Gross Margin % would have been 4% higher for the six months ended June 30, 2024.
Six Months Ended June 30, 2023(in millions)Commercial ProductsMCM ProductsServices Total SegmentContracts & Grants Total RevenuesRevenues$244.1 $201.5 $43.5 $489.1 $13.1 $502.2
Cost of sales or services 100.2 135.9 107.4 343.5
Gross margin$143.9 $65.6 $(63.9) $145.6 Gross margin % 59 % 33 %(147 )% 30 %
Add back:
Changes in fair value of contingent consideration$— $0.7 $— $0.7 Inventory step-up provision — 1.9 — 1.9 Restructuring costs — 2.0 — 2.0
Adjusted gross margin$143.9 $70.2 $(63.9) $150.2 Adjusted gross margin % 59 % 35 %(147 )% 31 %
Emergent BioSolutions, Inc.Reconciliation of Net Loss Forecast to Adjusted Net Loss Forecast ($ in millions)2024 Full Year ForecastSourceNet loss$(314) - $(274) Adjustments: Non-cash amortization charges$65Amortization of intangible assets, Other IncomeChanges in fair value of contingent consideration1Cost of MCM ProductsImpairments27Impairment of long-lived assetsSeverance and restructuring costs21Cost of MCM Products, Cost of Services, SG&A and R&DExit and disposal costs13R&DLoss (gain) on sale of business and assets held for sale40Other Income (Expense)Settlement charge, net110Cost of ServicesOther income (expense), net item3Other Income (Expense)Tax effect(81) Total adjustments:$199 Adjusted net loss$(115) - $(75)
Reconciliation of Net Loss Forecast to Adjusted EBITDA Forecast ($ in millions)2024 Full Year ForecastNet loss$(314) - $(274)Adjustments: Depreciation & amortization$111Income taxes46Total interest expense, net82Impairments27Changes in fair value of contingent consideration1Severance and restructuring costs21Exit and disposal costs13Loss (gain) on sale of business and assets held for sale40Settlement charge, net110Other income (expense), net item3Total adjustments$454Adjusted EBITDA$140 - $180
Reconciliations of Forecasted Total Revenues to Forecasted Total Segment Revenues and of Forecasted Segment and Total Segment Gross Margin and Gross Margin % to Forecasted Segment and Total Segment Adjusted Gross Margin and Adjusted Gross Margin %(1) (in millions)2024 Full Year Forecast Total revenues$1,050 - $1,125Contracts & Grants(25)Total segment revenues$1,025 - $1,100 Cost of sales or services$707 - $719Total segment gross margin$318 - $381Total segment gross margin %31% - 35%Add back: Changes in fair value of contingent consideration$1Settlement charge, net$110Restructuring costs$3Total segment adjusted gross margin$432 - $495Total segment adjusted gross margin %42% - 45%
Financial StatementVaccine
02 Jul 2024
GAITHERSBURG, Md., July 02, 2024 (GLOBE NEWSWIRE) -- Emergent BioSolutions Inc. (NYSE: EBS) today announced it has received more than $250 million in contract modifications from the Administration for Strategic Preparedness and Response (ASPR) at the United States Department of Health and Human Services (HHS), to deliver millions of doses of four medical countermeasures (MCMs). These contract modifications will help ensure continued supply/stockpiling of critical MCMs to address biological threats and emergencies against anthrax, smallpox and botulism. The four awards include: A contract modification valued at $30.0 million to supply CYFENDUS® (Anthrax Vaccine Adsorbed, Adjuvanted) this year. Previously known as AV7909, CYFENDUS® is a two-dose anthrax vaccine for post-exposure prophylaxis use in individuals 18 years of age and older. This new procurement funding is from Emergent’s existing 10-year contract with the Biomedical Advanced Research and Development Authority (BARDA) under contract (HHSO100201600030C).A contract modification valued at $99.9 million to supply ACAM2000® (Smallpox (Vaccinia) Vaccine, Live) this year. ACAM2000® is licensed for active immunization against smallpox disease for persons determined to be at high risk for smallpox infection. This is under Emergent’s existing 10-year contract with ASPR (75A50119C00071).Two new contract options totaling $122.9 million have been awarded to supply ASPR with VIGIV® [Vaccinia Immune Globulin Intravenous (Human)] drug product, and BAT® [Botulism Antitoxin Heptavalent (A, B, C, D, E, F, G) – (Equine)] drug substance and delivery of drug production this year and into early 2025. VIGIV® is used for treatment of complications to smallpox vaccination, while BAT® is indicated for the treatment of symptomatic botulism following documented or suspected exposure to botulinum neurotoxin serotypes A, B, C, D, E, F, or G in adults and pediatric patients. Both are under Emergent’s existing 10-year contracts with ASPR (75A50119C00037 and 75A50119C00075, respectively). “Securing multiple contract modifications with the U.S. government for our medical countermeasure products affirms that Emergent is a trusted biodefense partner, and also demonstrates the strength and sustainment of our product portfolio,” said Paul Williams, senior vice president, products head at Emergent. “As part of our longstanding public-private partnership, we stand ready to continue fulfilling preparedness priorities and stockpiling efforts in the U.S. and abroad.” Emergent specializes in developing, manufacturing, and supplying MCMs for military and civilian populations. The types and quantities of products that should be maintained in a stockpile will depend on the population requiring protection, the products available for meeting the threat, as well as government resources and priorities. About CYFENDUS® (Anthrax Vaccine Adsorbed, Adjuvanted) Indication CYFENDUS® (Anthrax Vaccine Adsorbed, Adjuvanted) is a vaccine indicated for post-exposure prophylaxis of anthrax disease following suspected or confirmed exposure to Bacillus anthracis in persons 18 through 65 years of age when given with recommended antibacterial drugs. The efficacy of CYFENDUS® vaccine for post-exposure prophylaxis (PEP) is based solely on studies in animal models of inhalational anthrax. Important Safety Information Contraindication: Do not take CYFENDUS® vaccine if you are allergic to CYFENDUS® vaccine, BioThrax® (Anthrax Vaccine Adsorbed) or any ingredient of the vaccine. Allergic reactions: Appropriate medical treatment and supervision must be available after receiving CYFENDUS® vaccine to manage possible serious allergic reactions. Get medical help right away if you have any symptoms of a serious allergic reaction. Altered Immunocompetence: Immunocompromised persons, including individuals receiving immunosuppressive therapy, may have a diminished immune response to CYFENDUS® vaccine. Pregnancy: CYFENDUS® vaccine can cause fetal harm when administered to a pregnant individual. Before getting CYFENDUS® vaccine, tell your healthcare provider if you may be pregnant, plan to get pregnant soon, or are nursing a baby. Adverse reactions: The most common adverse reactions reported were tenderness, pain, warmth, itching, swelling, redness, bruising, arm motion limitations, muscle aches, tiredness, headache, and fever. U.S. Prescribing InformationThe full Prescribing Information for CYFENDUS® vaccine can be found here. About ACAM2000® (Smallpox (Vaccinia) Vaccine, Live) ACAM2000® is the primary smallpox vaccine designated for use in a bioterrorism emergency, with doses having been supplied to the U.S. Strategic National Stockpile. ACAM2000® is also licensed in Australia and Singapore and is currently stockpiled both in the U.S. and internationally. ACAM2000® is indicated for active immunization against smallpox disease for persons determined to be at high risk for smallpox infection. The labeling for ACAM2000® contains a contraindication for individuals with severe immunodeficiency. Severe localized or systemic infection with vaccinia (progressive vaccinia) may occur in persons with weakened immune systems. Individuals with severe immunodeficiency who are not expected to benefit from the vaccine should not receive ACAM2000®. The risk for experiencing severe vaccination complications must be weighed against the risk for experiencing a potentially fatal smallpox infection. Additionally, there are warnings and precautions for myocarditis, pericarditis, encephalitis, encephalomyelitis, encephalopathy, generalized vaccinia, severe vaccinial skin infections, erythema multiforme major (including Stevens-Johnson Syndrome), eczema vaccinatum resulting in permanent sequelae or death, ocular complications; blindness and fetal death have occurred following either primary vaccination or revaccination with live vaccinia virus smallpox vaccines. These risks are increased in certain individuals and may result in severe disability, permanent neurological sequalae and/or death. Please see full Prescribing Information for full Boxed Warning and additional safety information. About VIGIV® [Vaccinia Immune Globulin Intravenous (Human)](See full prescribing information for complete boxed warning) WARNING: INTERACTIONS WITH GLUCOSE MONITORING SYSTEMSBlood glucose measurement in patients receiving Vaccinia Immune Globulin Intravenous (Human) (VIGIV) must be done with a glucose-specific method (monitor and test strips) to avoid interference by maltose contained in VIGIV. Maltose in IGIV products may give falsely high blood glucose levels in certain types of blood glucose testing systems (for example those based on the GDH-PQQ or glucose-dye-oxidoreductase methods) resulting in inappropriate administration of insulin and life-threatening hypoglycemia. Cases of true hypoglycemia may go untreated if the hypoglycemic state is masked by falsely elevated glucose readings. Carefully review the product information of the blood glucose testing system, including that of the test strips, to determine if the system is appropriate for use with maltose-containing parenteral products. VIGIV (vaccinia immune globulin intravenous, human) is an Immune Globulin (Human), 5% Liquid, indicated for the treatment and/or modification of the following conditions: eczema vaccinatum; progressive vaccinia; severe generalized vaccinia; vaccinia infections in individuals who have skin conditions such as burns, impetigo, varicella-zoster, or poison ivy; or in individuals who have eczematous skin lesions because of either the activity or extensiveness of such lesions; and aberrant infections induced by vaccinia virus that include its accidental implantation in eyes (except in cases of isolated keratitis), mouth, or other areas where vaccinia infection would constitute a special hazard.VIGIV is not considered to be effective in the treatment of postvaccinial encephalitis. VIGIV is contraindicated in: isolated vaccinia keratitis; Individuals with a history of anaphylaxis or prior severe systemic reaction associated with the parenteral administration of this or other human immune globulin preparations; IgA-deficient patients with antibodies against IgA and a history of IgA hypersensitivity, as it contains trace amounts of IgA (40 mcg/mL). Warnings and Precautions for VIGIV include: Hypersensitivity to human immune globulin (acute anaphylaxis)Acute renal dysfunction/failure. Use VIGIV with caution in patients with pre-existing renal insufficiency and in patients at increased risk of developing renal insufficiency.Thrombosis may occur with immune globulin products, including VIGIV. For patients at risk of thrombosis, administer VIGIV at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.Hemolysis or hemolytic anemiaAseptic meningitis syndrome (AMS)Noncardiogenic pulmonary edema [Transfusion-Related Acute Lung Injury (TRALI)]Transmission of infectious agents from human plasmaMonitor renal function and urine output in patients at risk of renal failure; check baseline blood viscosity in patients at risk of hyperviscosity; and conduct confirmatory tests if hemolysis or TRALI is suspected.Blood glucose monitoring There is no human or animal data for use of VIGIV during pregnancy. VIGIV should only be given to pregnant and nursing women if the potential benefits outweigh the potential risks. It is not known whether VIGIV is excreted in human milk. The safety and efficacy of VIGIV has not been established in pediatric and geriatric populations. The most frequently reported adverse reactions to VIGIV treatment in clinical trials (>10%) include: headache, nausea, rigors, and dizziness. Please see full Prescribing Information for VIGIV for additional safety information. About BAT® [Botulism Antitoxin Heptavalent (A, B, C, D, E, F, G) – (Equine)] BAT® is a mixture of immune globulin fragments indicated for the treatment of symptomatic botulism following documented or suspected exposure to botulinum neurotoxin serotypes A, B, C, D, E, F, or G in adults and pediatric patients. The effectiveness of BAT is based solely on efficacy studies conducted in animal models of botulism. The Warnings and Precautions for BAT include: Severe hypersensitivity reactions, including anaphylaxis, as well as delayed allergic reactions, including serum sickness may occur following BAT administration. Prepare for monitoring and management of allergic reactions. Infusion reactions. These reactions may be related to the infusion rate of BAT.Interference with blood glucose testing. Because BAT contains maltose, interference with non-glucose specific blood sugar testing systems can occur. Use glucose-specific testing systems.Transmissible infections agents. BAT is made from equine plasma and may contain infectious agents, e.g. viruses. There is no human or animal data for use of BAT during pregnancy. BAT should only be given to pregnant and nursing women if the potential benefits outweigh the potential risks. It is not known whether BAT is excreted in human milk. The safety and efficacy of BAT has not been established in pediatric and geriatric populations. Only limited safety data exists for pediatric populations. The most common adverse reactions observed in ≥5% of healthy volunteers in clinical trials were headache, nausea, pruritus, and urticaria. The most common adverse reactions reported in ≥1% of patients in a clinical study were pyrexia, rash, chills, nausea, and edema. One serious adverse reaction of hemodynamic instability was observed in one patient in the clinical study. Please see full Prescribing Information for BAT for additional safety information. About Emergent BioSolutions At Emergent, our mission is to protect and enhance life. For 25 years, we’ve been at work defending people from things we hope will never happen—so we are prepared just in case they ever do. We provide solutions for complex and urgent public health threats through a portfolio of vaccines and therapeutics that we develop and manufacture for governments and consumers. We also offer a range of integrated contract development and manufacturing services for pharmaceutical and biotechnology customers. To learn more about how we plan to protect or enhance 1 billion lives by 2030, visit our website and follow us on LinkedIn, X, Instagram, Apple Podcasts and Spotify. Safe Harbor StatementThis press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical fact, including statements regarding the development, availability, and government procurement of ACAM2000® vaccine, CYFENDUS® vaccine, BAT® and VIGIV® are forward-looking statements. We generally identify forward-looking statements by using words like “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “forecast,” “goal,” “intend,” “may,” “plan,” “should,” “will,” “would,” and similar expressions or variations thereof, or the negative thereof, but these terms are not the exclusive means of identifying such statements. Forward-looking statements are based on our current intentions, beliefs, and expectations regarding future events based on information that is currently available. We cannot guarantee that any forward-looking statement will be accurate. Readers should realize that if underlying assumptions prove inaccurate or if known or unknown risks or uncertainties materialize, actual results could differ materially from our expectations. Readers are, therefore, cautioned not to place undue reliance on any forward-looking statement. Any forward-looking statement speaks only as of the date of this press release, and, except as required by law, we do not undertake to update any forward-looking statement to reflect new information, events, or circumstances. Readers should consider this cautionary statement, as well as the risk factors identified in our periodic reports filed with the U.S. Securities and Exchange Commission, when evaluating our forward-looking statements. Investor Contact:Richard S. LindahlExecutive Vice President, CFOlindahlr@ebsi.com Media Contact:Assal HellmerVice President, Communicationsmediarelations@ebsi.com
Vaccine
02 Jul 2024
GAITHERSBURG, Md., July 02, 2024 (GLOBE NEWSWIRE) -- Emergent BioSolutions Inc. (NYSE: EBS) today announced it has received more than $250 million in contract modifications from the Administration for Strategic Preparedness and Response (ASPR) at the United States Department of Health and Human Services (HHS), to deliver millions of doses of four medical countermeasures (MCMs). These contract modifications will help ensure continued supply/stockpiling of critical MCMs to address biological threats and emergencies against anthrax, smallpox and botulism. The four awards include: A contract modification valued at $30.0 million to supply CYFENDUS® (Anthrax Vaccine Adsorbed, Adjuvanted) this year. Previously known as AV7909, CYFENDUS® is a two-dose anthrax vaccine for post-exposure prophylaxis use in individuals 18 years of age and older. This new procurement funding is from Emergent’s existing 10-year contract with the Biomedical Advanced Research and Development Authority (BARDA) under contract (HHSO100201600030C).A contract modification valued at $99.9 million to supply ACAM2000® (Smallpox (Vaccinia) Vaccine, Live) this year. ACAM2000® is licensed for active immunization against smallpox disease for persons determined to be at high risk for smallpox infection. This is under Emergent’s existing 10-year contract with ASPR (75A50119C00071).Two new contract options totaling $122.9 million have been awarded to supply the Strategic National Stockpile with VIGIV® [Vaccinia Immune Globulin Intravenous (Human)] drug product, and BAT® [Botulism Antitoxin Heptavalent (A, B, C, D, E, F, G) – (Equine)] drug substance and delivery of drug production this year and into early 2025. VIGIV® is used for treatment of complications to smallpox vaccination, while BAT® is indicated for the treatment of symptomatic botulism following documented or suspected exposure to botulinum neurotoxin serotypes A, B, C, D, E, F, or G in adults and pediatric patients. Both are under Emergent’s existing 10-year contracts with ASPR (75A50119C00037 and 75A50119C00075, respectively). “Securing multiple contract modifications with the U.S. government for our medical countermeasure products affirms that Emergent is a trusted biodefense partner, and also demonstrates the strength and sustainment of our product portfolio,” said Paul Williams, senior vice president, products head at Emergent. “As part of our longstanding public-private partnership, we stand ready to continue fulfilling preparedness priorities and stockpiling efforts in the U.S. and abroad.” Emergent specializes in developing, manufacturing, and supplying MCMs for military and civilian populations. The types and quantities of products that should be maintained in a stockpile will depend on the population requiring protection, the products available for meeting the threat, as well as government resources and priorities. About CYFENDUS® (Anthrax Vaccine Adsorbed, Adjuvanted) Indication CYFENDUS® (Anthrax Vaccine Adsorbed, Adjuvanted) is a vaccine indicated for post-exposure prophylaxis of anthrax disease following suspected or confirmed exposure to Bacillus anthracis in persons 18 through 65 years of age when given with recommended antibacterial drugs. The efficacy of CYFENDUS® vaccine for post-exposure prophylaxis (PEP) is based solely on studies in animal models of inhalational anthrax. Important Safety Information Contraindication: Do not take CYFENDUS® vaccine if you are allergic to CYFENDUS® vaccine, BioThrax® (Anthrax Vaccine Adsorbed) or any ingredient of the vaccine. Allergic reactions: Appropriate medical treatment and supervision must be available after receiving CYFENDUS® vaccine to manage possible serious allergic reactions. Get medical help right away if you have any symptoms of a serious allergic reaction. Altered Immunocompetence: Immunocompromised persons, including individuals receiving immunosuppressive therapy, may have a diminished immune response to CYFENDUS® vaccine. Pregnancy: CYFENDUS® vaccine can cause fetal harm when administered to a pregnant individual. Before getting CYFENDUS® vaccine, tell your healthcare provider if you may be pregnant, plan to get pregnant soon, or are nursing a baby. Adverse reactions: The most common adverse reactions reported were tenderness, pain, warmth, itching, swelling, redness, bruising, arm motion limitations, muscle aches, tiredness, headache, and fever. U.S. Prescribing InformationThe full Prescribing Information for CYFENDUS® vaccine can be found here. About ACAM2000® (Smallpox (Vaccinia) Vaccine, Live) ACAM2000® is the primary smallpox vaccine designated for use in a bioterrorism emergency, with doses having been supplied to the U.S. Strategic National Stockpile. ACAM2000® is also licensed in Australia and Singapore and is currently stockpiled both in the U.S. and internationally. ACAM2000® is indicated for active immunization against smallpox disease for persons determined to be at high risk for smallpox infection. The labeling for ACAM2000® contains a contraindication for individuals with severe immunodeficiency. Severe localized or systemic infection with vaccinia (progressive vaccinia) may occur in persons with weakened immune systems. Individuals with severe immunodeficiency who are not expected to benefit from the vaccine should not receive ACAM2000®. The risk for experiencing severe vaccination complications must be weighed against the risk for experiencing a potentially fatal smallpox infection. Additionally, there are warnings and precautions for myocarditis, pericarditis, encephalitis, encephalomyelitis, encephalopathy, generalized vaccinia, severe vaccinial skin infections, erythema multiforme major (including Stevens-Johnson Syndrome), eczema vaccinatum resulting in permanent sequelae or death, ocular complications; blindness and fetal death have occurred following either primary vaccination or revaccination with live vaccinia virus smallpox vaccines. These risks are increased in certain individuals and may result in severe disability, permanent neurological sequalae and/or death. Please see full Prescribing Information for full Boxed Warning and additional safety information. About VIGIV® [Vaccinia Immune Globulin Intravenous (Human)](See full prescribing information for complete boxed warning) WARNING: INTERACTIONS WITH GLUCOSE MONITORING SYSTEMSBlood glucose measurement in patients receiving Vaccinia Immune Globulin Intravenous (Human) (VIGIV) must be done with a glucose-specific method (monitor and test strips) to avoid interference by maltose contained in VIGIV. Maltose in IGIV products may give falsely high blood glucose levels in certain types of blood glucose testing systems (for example those based on the GDH-PQQ or glucose-dye-oxidoreductase methods) resulting in inappropriate administration of insulin and life-threatening hypoglycemia. Cases of true hypoglycemia may go untreated if the hypoglycemic state is masked by falsely elevated glucose readings. Carefully review the product information of the blood glucose testing system, including that of the test strips, to determine if the system is appropriate for use with maltose-containing parenteral products. VIGIV (vaccinia immune globulin intravenous, human) is an Immune Globulin (Human), 5% Liquid, indicated for the treatment and/or modification of the following conditions: eczema vaccinatum; progressive vaccinia; severe generalized vaccinia; vaccinia infections in individuals who have skin conditions such as burns, impetigo, varicella-zoster, or poison ivy; or in individuals who have eczematous skin lesions because of either the activity or extensiveness of such lesions; and aberrant infections induced by vaccinia virus that include its accidental implantation in eyes (except in cases of isolated keratitis), mouth, or other areas where vaccinia infection would constitute a special hazard.VIGIV is not considered to be effective in the treatment of postvaccinial encephalitis. VIGIV is contraindicated in: isolated vaccinia keratitis; Individuals with a history of anaphylaxis or prior severe systemic reaction associated with the parenteral administration of this or other human immune globulin preparations; IgA-deficient patients with antibodies against IgA and a history of IgA hypersensitivity, as it contains trace amounts of IgA (40 mcg/mL). Warnings and Precautions for VIGIV include: Hypersensitivity to human immune globulin (acute anaphylaxis)Acute renal dysfunction/failure. Use VIGIV with caution in patients with pre-existing renal insufficiency and in patients at increased risk of developing renal insufficiency.Thrombosis may occur with immune globulin products, including VIGIV. For patients at risk of thrombosis, administer VIGIV at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.Hemolysis or hemolytic anemiaAseptic meningitis syndrome (AMS)Noncardiogenic pulmonary edema [Transfusion-Related Acute Lung Injury (TRALI)]Transmission of infectious agents from human plasmaMonitor renal function and urine output in patients at risk of renal failure; check baseline blood viscosity in patients at risk of hyperviscosity; and conduct confirmatory tests if hemolysis or TRALI is suspected.Blood glucose monitoring There is no human or animal data for use of VIGIV during pregnancy. VIGIV should only be given to pregnant and nursing women if the potential benefits outweigh the potential risks. It is not known whether VIGIV is excreted in human milk. The safety and efficacy of VIGIV has not been established in pediatric and geriatric populations. The most frequently reported adverse reactions to VIGIV treatment in clinical trials (>10%) include: headache, nausea, rigors, and dizziness. Please see full Prescribing Information for VIGIV for additional safety information. About BAT® [Botulism Antitoxin Heptavalent (A, B, C, D, E, F, G) – (Equine)] BAT® is a mixture of immune globulin fragments indicated for the treatment of symptomatic botulism following documented or suspected exposure to botulinum neurotoxin serotypes A, B, C, D, E, F, or G in adults and pediatric patients. The effectiveness of BAT is based solely on efficacy studies conducted in animal models of botulism. The Warnings and Precautions for BAT include: Severe hypersensitivity reactions, including anaphylaxis, as well as delayed allergic reactions, including serum sickness may occur following BAT administration. Prepare for monitoring and management of allergic reactions. Infusion reactions. These reactions may be related to the infusion rate of BAT.Interference with blood glucose testing. Because BAT contains maltose, interference with non-glucose specific blood sugar testing systems can occur. Use glucose-specific testing systems.Transmissible infections agents. BAT is made from equine plasma and may contain infectious agents, e.g. viruses. There is no human or animal data for use of BAT during pregnancy. BAT should only be given to pregnant and nursing women if the potential benefits outweigh the potential risks. It is not known whether BAT is excreted in human milk. The safety and efficacy of BAT has not been established in pediatric and geriatric populations. Only limited safety data exists for pediatric populations. The most common adverse reactions observed in ≥5% of healthy volunteers in clinical trials were headache, nausea, pruritus, and urticaria. The most common adverse reactions reported in ≥1% of patients in a clinical study were pyrexia, rash, chills, nausea, and edema. One serious adverse reaction of hemodynamic instability was observed in one patient in the clinical study. Please see full Prescribing Information for BAT for additional safety information. About Emergent BioSolutions At Emergent, our mission is to protect and enhance life. For 25 years, we’ve been at work defending people from things we hope will never happen—so we are prepared just in case they ever do. We provide solutions for complex and urgent public health threats through a portfolio of vaccines and therapeutics that we develop and manufacture for governments and consumers. We also offer a range of integrated contract development and manufacturing services for pharmaceutical and biotechnology customers. To learn more about how we plan to protect or enhance 1 billion lives by 2030, visit our website and follow us on LinkedIn, X, Instagram, Apple Podcasts and Spotify. Safe Harbor StatementThis press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical fact, including statements regarding the development, availability, and government procurement of ACAM2000® vaccine, CYFENDUS® vaccine, BAT® and VIGIV® are forward-looking statements. We generally identify forward-looking statements by using words like “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “forecast,” “goal,” “intend,” “may,” “plan,” “should,” “will,” “would,” and similar expressions or variations thereof, or the negative thereof, but these terms are not the exclusive means of identifying such statements. Forward-looking statements are based on our current intentions, beliefs, and expectations regarding future events based on information that is currently available. We cannot guarantee that any forward-looking statement will be accurate. Readers should realize that if underlying assumptions prove inaccurate or if known or unknown risks or uncertainties materialize, actual results could differ materially from our expectations. Readers are, therefore, cautioned not to place undue reliance on any forward-looking statement. Any forward-looking statement speaks only as of the date of this press release, and, except as required by law, we do not undertake to update any forward-looking statement to reflect new information, events, or circumstances. Readers should consider this cautionary statement, as well as the risk factors identified in our periodic reports filed with the U.S. Securities and Exchange Commission, when evaluating our forward-looking statements. Investor Contact:Richard S. LindahlExecutive Vice President, CFOlindahlr@ebsi.com Media Contact:Assal HellmerVice President, Communicationsmediarelations@ebsi.com
Vaccine
100 Deals associated with Heptavalent combination vaccine(GlaxoSmithKline Plc)
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Haemophilus Infections | Phase 2 | SK | 01 Apr 2009 | |
| Haemophilus Infections | Phase 2 | SK | 01 Apr 2009 | |
| Meningitis | Phase 2 | SK | 01 Apr 2009 | |
| Meningitis | Phase 2 | SK | 01 Apr 2009 | |
| Poliomyelitis | Phase 2 | SK | 01 Apr 2009 | |
| Poliomyelitis | Phase 2 | SK | 01 Apr 2009 | |
| Tetanus | Phase 2 | SK | 01 Apr 2009 | |
| Tetanus | Phase 2 | SK | 01 Apr 2009 | |
| Whooping Cough | Phase 2 | SK | 01 Apr 2009 | |
| Whooping Cough | Phase 2 | SK | 01 Apr 2009 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 2 | 421 | (GSK2202083A + Synflorix Group) | plieswthgr(vnmbmoqfqh) = kuyvnlvvji jcglyjpwee (jvhftbiyfm, rrbhoratof - jfdcxooorh) View more | - | 14 Apr 2017 | ||
(Infanrix Hexa + Menjugate Group) | plieswthgr(vnmbmoqfqh) = nqspsiqamt jcglyjpwee (jvhftbiyfm, mvkxgjljno - hdqoukyvbb) View more | ||||||
Phase 2 | 391 | (GSK2202083A + Synflorix Group) | lrimntzvdd(bmzuvdyyjk) = apkchqenky dwcgbzamlf (ivcbrrgwbh, uovakppylv - qjncznwqhi) View more | - | 11 Apr 2017 | ||
(Infanrix Hexa/Menjugate Group) | lrimntzvdd(bmzuvdyyjk) = nxldhtwsyo dwcgbzamlf (ivcbrrgwbh, wroqwlbnhs - apmnjknqlg) View more | ||||||
Phase 2 | 16 | (GSK2202083A Group) | tosbkbgnye(pmlfpzjxoc) = hecjkvtita xrlgszsfgw (iuehdghwrw, aunezisslt - vjsofogphc) View more | - | 04 Apr 2017 | ||
(Infanrix + Menjugate Group) | tosbkbgnye(pmlfpzjxoc) = yiyrpebpya xrlgszsfgw (iuehdghwrw, avkihobyby - ywgjzwbfby) View more | ||||||
Phase 2 | 480 | (GSK2202083A Group) | takeulgspf(nupmpauedg) = llxcizgnat grpbtohfzy (pkkkferaei, bvkextlssy - lbucuhdhjo) View more | - | 15 Aug 2014 | ||
(Infanrix Hexa Group) | takeulgspf(nupmpauedg) = jykpqmdcmz grpbtohfzy (pkkkferaei, gpqvrzwzvp - ziuxgbmagm) View more | ||||||
Not Applicable | Acute otitis media Streptococcus Pneumoniae | Haemophilus Influenzae | 832 | Heptavalent Pneumococcal Conjugate Vaccine | (zaftjzbnme): odds ratio = 0.4 (95% CI, 0.22 - 0.75) View more | - | 01 May 2014 | |
Heptavalent Pneumococcal Conjugate Vaccine |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
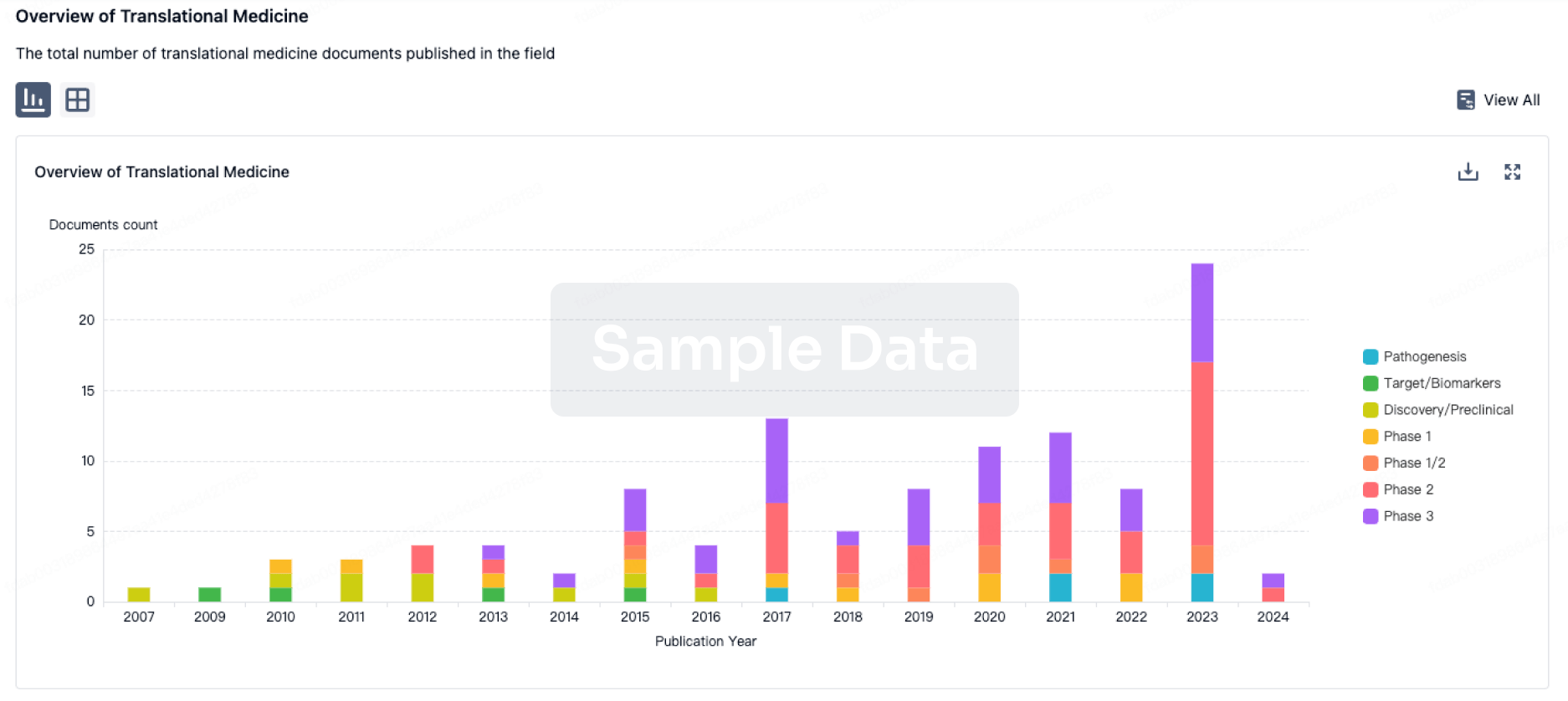
Deal
Boost your decision using our deal data.
login
or
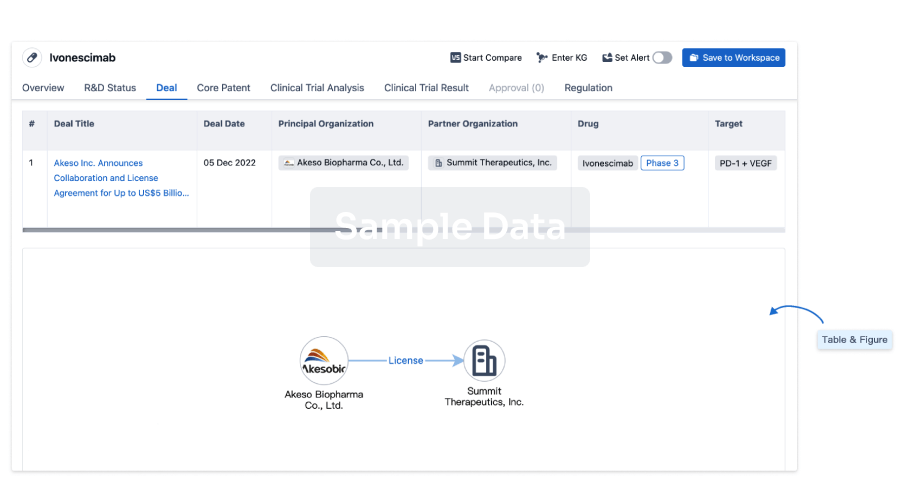
Core Patent
Boost your research with our Core Patent data.
login
or
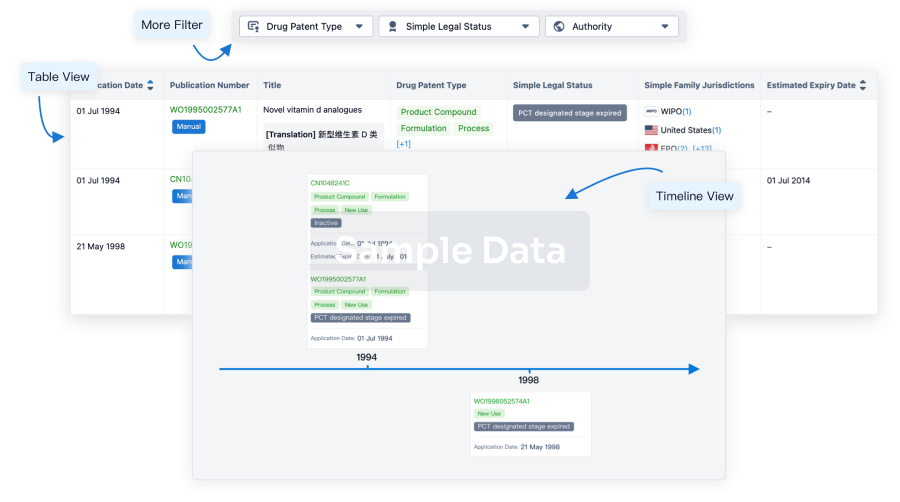
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
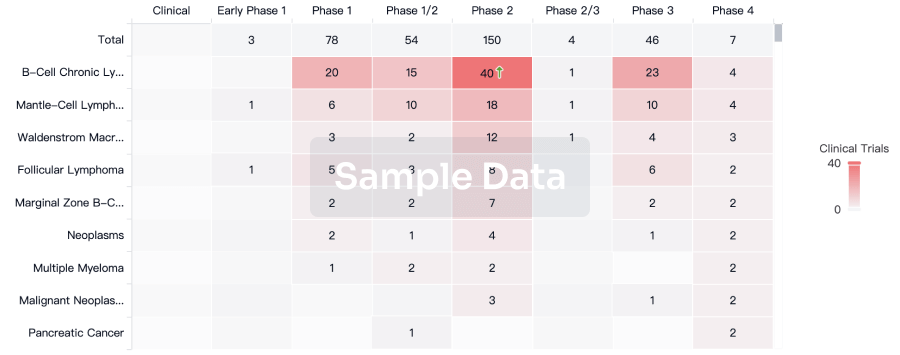
Approval
Accelerate your research with the latest regulatory approval information.
login
or
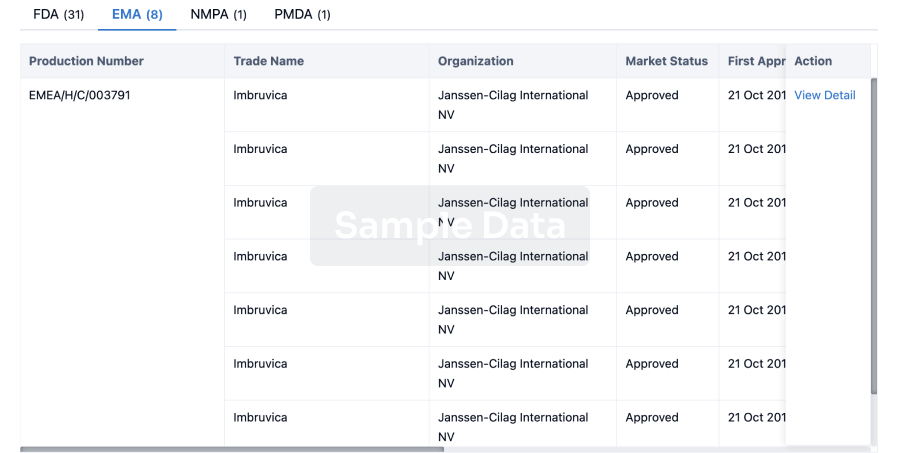
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
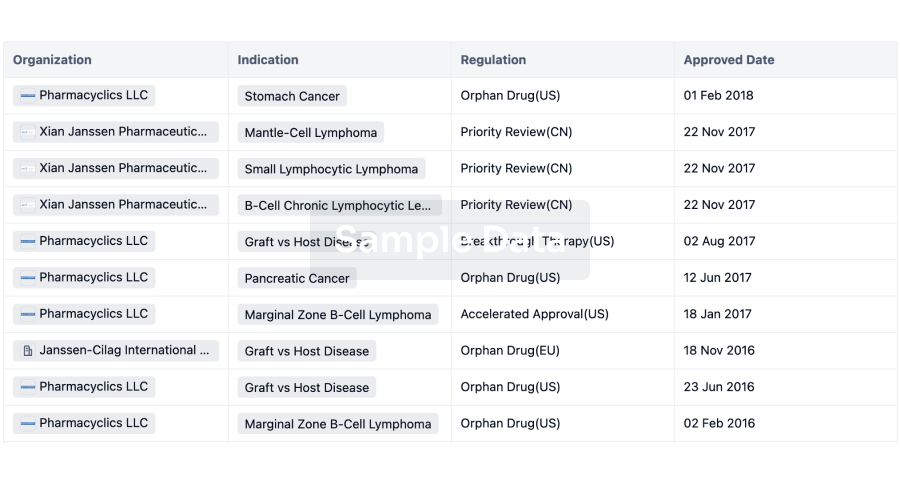
Chat with Hiro
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
