Request Demo
Last update 17 May 2025
evoncabtagene pazurgedleucel
Last update 17 May 2025
Overview
Basic Info
Drug Type Universal CAR-T |
Synonyms Anti-CD19+ CAR-T cells (CRISPR Therapeutics), CTX 101, CTX 110 + [2] |
Target |
Action inhibitors |
Mechanism CD19 inhibitors(B-lymphocyte antigen CD19 inhibitors), Gene transference(Gene transference), T lymphocyte replacements |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
License Organization- |
Drug Highest PhaseDiscontinuedPhase 1/2 |
First Approval Date- |
RegulationRegenerative Medicine Advanced Therapy (United States) |
Login to view timeline
Structure/Sequence
Sequence Code 133348ScFv

Target: *****
Sequence Code 10019466CAR construct

Target: *****
Related
1
Clinical Trials associated with evoncabtagene pazurgedleucelNCT04035434
A Phase 1/2 Dose Escalation and Cohort Expansion Study of the Safety and Efficacy of Allogeneic CRISPR-Cas9-Engineered T Cells (CTX110) in Subjects With Relapsed or Refractory B-Cell Malignancies (CARBON)
This is an open-label, multicenter, Phase 1/2 study evaluating the safety and efficacy of CTX110 in subjects with relapsed or refractory B-cell malignancies.
Start Date22 Jul 2019 |
Sponsor / Collaborator |
100 Clinical Results associated with evoncabtagene pazurgedleucel
Login to view more data
100 Translational Medicine associated with evoncabtagene pazurgedleucel
Login to view more data
100 Patents (Medical) associated with evoncabtagene pazurgedleucel
Login to view more data
3
Literatures (Medical) associated with evoncabtagene pazurgedleucel01 Jul 2023·Nature cancer
Tumor-intrinsic sensitivity to the pro-apoptotic effects of IFN-γ is a major determinant of CD4+ CAR T-cell antitumor activity.
Article
Author: Bousso, Philippe ; Cazaux, Marine ; Corre, Béatrice ; Thieblemont, Catherine ; Grandjean, Capucine L ; Cuffel, Alexis ; Alonso, Ruby ; Guerin, Marion V ; Lemaître, Fabrice ; Boulch, Morgane ; Caillat-Zucman, Sophie ; Beer, Alexander ; Menger, Laurie ; Morin, Florence ; Garcia, Zacarias
CD4+ T cells and CD4+ chimeric antigen receptor (CAR) T cells display highly variable antitumor activity in preclinical models and in patients; however, the mechanisms dictating how and when CD4+ T cells promote tumor regression are incompletely understood. With the help of functional intravital imaging, we report that interferon (IFN)-γ production but not perforin-mediated cytotoxicity was the dominant mechanism for tumor elimination by anti-CD19 CD4+ CAR T cells. Mechanistically, mouse or human CD4+ CAR T-cell-derived IFN-γ diffused extensively to act on tumor cells at distance selectively killing tumors sensitive to cytokine-induced apoptosis, including antigen-negative variants. In anti-CD19 CAR T-cell-treated patients exhibiting elevated CAR CD4:CD8 ratios, strong induction of serum IFN-γ was associated with increased survival. We propose that the sensitivity of tumor cells to the pro-apoptotic activity of IFN-γ is a major determinant of CD4+ CAR T-cell efficacy and may be considered to guide the use of CD4+ T cells during immunotherapy.
01 Feb 2013·Leukemia & lymphomaQ4 · MEDICINE
Efficacy and safety of adoptive immunotherapy using anti-CD19 chimeric antigen receptor transduced T-cells: a systematic review of phase I clinical trials
Q4 · MEDICINE
Review
Author: Tang, Yong-Min ; Xu, Xiao-Jun ; Zhao, Hai-Zhao
There remain some key questions regarding the adoptive infusion of chimeric antigen receptor (CAR) transduced T-cells in the clinical setting. This article systematically reviews the phase I clinical trials using CARs targeting CD19 in B-lineage malignancies. Twenty-nine patients were enrolled and the 6-month progression free survival for this cohort was 50.0 ± 9.9%. Univariate analysis showed that patients benefited from lymphodepletion before CAR+T-cell infusion and the administration of interleukin-2 (IL-2). Longer-term persistence (≥ 4 weeks) and stronger expansion of CAR+ T-cells in the blood and higher peak serum interferon-γ (IFN-γ) level (≥ 200 pg/mL) were also related to superior outcome. Regarding treatment-related adverse events, the most prominent toxicities were fever, rigors, chills, acute renal failure, hypotension and capillary leak syndrome. In conclusion, anti-CD19 CAR+ T-cells have shown some benefits in patients with B-lineage malignancies and are well tolerated in most patients. Preconditioning and cytokine supplement are required to improve the clinical outcome.
Experimental Hematology & Oncology
Addressing graft-versus-host disease in allogeneic cell-based immunotherapy for cancer
Review
Author: Fang, Ying ; Yang, Lili ; Li, Yan-Ruide ; Lyu, Zibai ; Chen, Yuning ; Niu, Siyue
Abstract:
Allogeneic cell-based immunotherapies, particularly CAR-T cell therapy, represent a significant advancement in cancer treatment, offering scalable and consistent alternatives to autologous therapies. However, their widespread use is limited by the risk of graft-versus-host disease (GvHD). This review provides a comprehensive overview of GvHD in the context of allogeneic cell-based cancer immunotherapy and evaluates current strategies to mitigate its effects. Key strategies include genetic engineering approaches such as T cell receptor (TCR) knockout (KO) and T cell receptor alpha constant (TRAC) CAR knock-in. Alternative immune cell types like natural killer (NK) cells and natural killer T (NKT) cells offer potential solutions due to their lower alloreactivity. Additionally, stem cell technology, utilizing induced pluripotent stem cells (iPSCs), enables standardized and scalable production of engineered CAR-T cells. Clinical trials evaluating these strategies, such as UCART19 and CTX110, demonstrate promising results in preventing GvHD while maintaining anti-tumor efficacy. The review also addresses manufacturing considerations for allogeneic cell products and the challenges in translating preclinical findings into clinical success. By addressing these challenges, allogeneic cell-based immunotherapy continues to advance, paving the way for more accessible, scalable, and effective cancer treatments.
35
News (Medical) associated with evoncabtagene pazurgedleucel09 Dec 2024
-Preliminary data demonstrate that CTX112™ has the potential to provide meaningful clinical benefit with a well-tolerated safety profile using a standard lymphodepletion protocol- -Company announced that the U.S. Food and Drug Administration (FDA) granted Regenerative Medicine Advanced Therapy (RMAT) designation to CTX112 for the treatment of certain relapsed or refractory (R/R) CD19-positive B-cell malignancies- -CTX112 is also in a Phase 1 clinical trial in systemic lupus erythematosus (SLE), with the potential to expand into additional autoimmune indications in the future- -Company expects to provide a broader update across oncology and autoimmune indications in mid-2025- ZUG, Switzerland and BOSTON, Dec. 09, 2024 (GLOBE NEWSWIRE) -- CRISPR Therapeutics (Nasdaq: CRSP), a biopharmaceutical company focused on creating transformative gene-based medicines for serious diseases, today presented data from the Company’s ongoing Phase 1/2 dose escalation clinical trial evaluating the safety and efficacy of CTX112™, a next-generation CD19 allogeneic CAR T cell therapy, in relapsed or refractory (R/R) CD19-positive B-cell malignancies at the 2024 American Society of Hematology (ASH) Annual Meeting. Additionally, the Company announced that the U.S. Food and Drug Administration (FDA) granted Regenerative Medicine Advanced Therapy (RMAT) designation to CTX112 for the treatment of R/R follicular lymphoma and marginal zone lymphoma. “We are excited by these encouraging results on safety and efficacy for CTX112, which demonstrate the potential of an allogeneic CAR T treatment to produce complete remissions in heavily pre-treated patients,” said Naimish Patel, M.D., Chief Medical Officer of CRISPR Therapeutics. “The data support a well-tolerated safety profile and the possibility to address the unmet need in this patient population with an off-the-shelf CAR T therapy. These results also support the potential treatment of certain autoimmune diseases by CTX112, and we are continuing to advance our SLE trial. We would like to extend our deepest gratitude to the patients, their families, and the investigators who have participated in our clinical trials. Their dedication and contributions are invaluable to advancing our programs and bringing us closer to potentially innovative treatments.” “We are very encouraged by the progress and early clinical data from CTX112, which could result in better outcomes for patients,” said Armin Ghobadi, M.D., Professor of Medicine and Clinical Director, Center for Gene and Cellular Immunotherapy (CGCI), at Washington University School of Medicine. “CTX112 has shown dose-dependent efficacy and response rates that are comparable to the early autologous CAR T trials. These early results highlight the potential for CTX112 to emerge as an effective, off-the-shelf CAR T therapy for patients with relapsed or refractory CD19-positive B-cell malignancies.” CTX112 Trial OverviewThe Phase 1/2 clinical trial is an open-label, multicenter study evaluating the safety and efficacy of CTX112 in relapsed or refractory (R/R) B-cell malignancies. Eligible disease subtypes include large B-cell lymphoma (LBCL), follicular lymphoma (FL) grade 1-3a, marginal zone lymphoma (MZL), and mantle cell lymphoma (MCL). CTX112 was infused after a standard course of lymphodepleting chemotherapy (3 days of 30 mg/m2 fludarabine and 500 mg/m2 cyclophosphamide). Data were presented from 12 subjects treated during the dose escalation with CTX112 doses ranging from 30 x 106 (Dose Level [DL] 1) to 600 x 106 (DL4) CAR+ T cells. The study population was enriched for patients with high-risk characteristics, including: 1) primary refractory disease or early relapse to first-line therapy (75%); 2) high tumor burden (SPD > 4000 mm2, 50%); and 3) high disease prognostic index score (IPI, FLIPI, MZL-IPI ≥3) or elevated lactate dehydrogenase (75%). SafetyCTX112 was well tolerated across all dose levels. The adverse events of interest are shown in the table below. There were no reported dose limiting toxicities (DLTs) and no reported Grade ≥3 infections. All grade 3 or 4 cytopenias (i.e., neutropenia, thrombocytopenia, anemia) following lymphodepleting chemotherapy resolved to Grade 2 or better within 1 month of CTX112 infusion. There were no reported cases of Graft versus Host Disease (GvHD),All cases of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were Grade 1 or 2 per the American Society for Transplantation and Cellular Therapy (ASTCT) criteria. These low-grade CRS and ICANS events followed standard toxicity management protocols. Table 1. Summary of Adverse Events of Interest Cell dose (CAR+ T cells)DL130x106 N=3DL2100x106 N=3DL3300x106 N=3DL4600x106 N=3TotalN=12Gr1-2Gr ≥3Gr1-2Gr ≥3Gr1-2Gr ≥3Gr1-2Gr ≥3Gr1-2Gr ≥3CRS, n (%)1 (33)02 (67)01 (33)03 (100)07 (58)0ICANS, n (%)001 (33)01 (33)02 (67)04 (33)0Infections, n (%)1 (33)0002 (67)02 (67)05 (42)0 Clinical EfficacyCTX112 produced responses at all dose levels. Disease assessment was performed by investigator review according to the Lugano criteria. Table 2. Summary of Clinical Efficacy Cell dose(CAR+ T cells)DL130x106N=3DL2100x106N=3DL3300x106N=3DL4600x106N=3TotalN=12Objective Response Rate (ORR), n (%)2 (67)2 (67)2 (67)2 (67)8 (67)Complete Response Rate (CRR), n (%)1 (33)2 (67)1 (33)2 (67)6 (50)Partial Response Rate, n (%)1 (33)01 (33)02 (17)
Objective and complete responses were seen at all dose levels and in all treated NHL subtypes (i.e., FL, MZL, MCL and LBCL).Responses were also seen in patients with poor prognostic factors including primary refractory disease, early relapse, and high baseline tumor burden (e.g., SPD > 4000 mm2).Five patients (of the 12 treated) have achieved responses lasting for more than 6 months, including one patient whose 6-month response was confirmed after the data cut-off date. One patient treated at DL1 remains in complete remission over a year after initial CTX112 infusion. The clinical efficacy of CTX112 is supported by a clearly differentiated pharmacokinetic profile for an allogeneic CAR T cell therapy.The mean peak concentration and total exposure were significantly higher at DL3 and DL4 vs. DL1 and DL2. This dose dependence suggests the possibility of deeper and more durable responses as the trial moves from dose escalation to dose optimization.Comparing DL3, the addition of Regnase-1 and TGFβR2 edits results in 7-fold higher peak concentration (Cmax) and 9.7-fold higher mean area under the curve (AUC) for CTX112 relative to CTX110®. Furthermore, at DL4, both Cmax and AUC are showing significantly more consistent and predictable increases. This suggests that the novel CRISPR/Cas9 potency edits are leading to higher CAR T cell expansion and functional persistence without enhanced or increased lymphodepleting chemotherapy doses. These preliminary data demonstrate that CTX112 has the potential to provide meaningful clinical benefit with a well-tolerated safety profile. Given the inherent difficulties of manufacturing a CAR T therapy from a patient’s own diseased cells, allogeneic cellular therapy approaches have greater potential to address the unmet need in this patient population. These promising findings underscore the potential of allogeneic cell therapies to offer a transformative option for patients, and we remain committed to advancing this innovative approach to address the significant unmet medical need in this area. Regenerative Medicine Advanced Therapy (RMAT) DesignationEstablished under the 21st Century Cures Act, RMAT designation is a dedicated program designed to expedite the drug development and review processes for promising regenerative medicine pipeline products. A regenerative medicine therapy is eligible for RMAT designation if it is a cell therapy, therapeutic tissue engineering product, human cell and tissue product or any combination product of such therapies that is intended to treat, modify, reverse or cure a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug or therapy has the potential to address unmet medical needs for such disease or condition. Similar to Breakthrough Therapy designation, RMAT designation provides the benefits of intensive FDA guidance on efficient drug development, including the ability for early interactions with FDA to discuss surrogate or intermediate endpoints, potential ways to support accelerated approval and satisfy post-approval requirements, potential priority review of the biologics license application (BLA) and other opportunities to expedite development and review. About CTX112CTX112 is a next-generation, wholly-owned, allogeneic CAR T product candidate targeting Cluster of Differentiation 19, or CD19, which incorporates edits designed to evade the immune system, enhance CAR T potency and reduce CAR T exhaustion. CTX112 is being investigated in an ongoing clinical trial designed to assess safety and efficacy of the product candidate in adult patients with relapsed or refractory CD19-positive B-cell malignancies who have received at least two prior lines of therapy. In addition, CTX112 is being investigated in an ongoing clinical trial designed to assess safety and efficacy of the product candidate in adult patients with system lupus erythematosus. About CRISPR TherapeuticsSince its inception over a decade ago, CRISPR Therapeutics has transformed from a research-stage company advancing programs in the field of gene editing, to a company that celebrated the historic approval of the first-ever CRISPR-based therapy in 2023 and has a diverse portfolio of product candidates across a broad range of disease areas including hemoglobinopathies, oncology, regenerative medicine, cardiovascular, autoimmune, and rare diseases. CRISPR Therapeutics advanced the first-ever CRISPR/Cas9 gene-edited therapy into the clinic in 2018 to investigate the treatment of sickle cell disease or transfusion-dependent beta thalassemia, and beginning in late 2023, CASGEVY™ (exagamglogene autotemcel [exa-cel]) was approved in some countries to treat eligible patients with either of those conditions. The Nobel Prize-winning CRISPR science has revolutionized biomedical research and represents a powerful, clinically validated approach with the potential to create a new class of potentially transformative medicines. To accelerate and expand its efforts, CRISPR Therapeutics has established strategic partnerships with leading companies including Bayer and Vertex Pharmaceuticals. CRISPR Therapeutics AG is headquartered in Zug, Switzerland, with its wholly-owned U.S. subsidiary, CRISPR Therapeutics, Inc., and R&D operations based in Boston, Massachusetts and San Francisco, California, and business offices in London, United Kingdom. To learn more, visit www.crisprtx.com. CRISPR THERAPEUTICS® standard character mark and design logo, CTX110® and CTX112™ are trademarks and registered trademarks of CRISPR Therapeutics AG. The CASGEVY™ word mark and design are trademarks of Vertex Pharmaceuticals Incorporated. All other trademarks and registered trademarks are the property of their respective owners. CRISPR Special Note Regarding Forward-Looking StatementStatements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding any or all of the following: (i) CRISPR Therapeutics’ preclinical studies, clinical trials and pipeline products and programs, including, without limitation, manufacturing capabilities, status of such studies and trials, potential expansion into new indications and expectations regarding data, safety and efficacy generally; (ii) the clinical data being presented at the 2024 ASH Annual Meeting; (iii) discussions with regulatory authorities related to product candidates under development by CRISPR Therapeutics including, without limitation, expectations regarding the benefits of RMAT designation; and (iv) the therapeutic value, development, and commercial potential of gene editing technologies and therapies, including CRISPR/Cas9. Risks that contribute to the uncertain nature of the forward-looking statements include, without limitation, the risks and uncertainties discussed under the heading “Risk Factors” in its most recent annual report on Form 10-K and in any other subsequent filings made by CRISPR Therapeutics with the U.S. Securities and Exchange Commission. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date they are made. The Company disclaims any obligation or undertaking to update or revise any forward-looking statements contained in this press release other than to the extent required by law. This press release discusses CRISPR/Cas9 gene editing investigational therapies and is not intended to convey conclusions about efficacy or safety as to those investigational therapies or uses of such investigational therapies. There is no guarantee that any investigational therapy will successfully complete clinical development or gain approval from applicable regulatory authorities. Investor Contact:Susan Kim+1-617-307-7503susan.kim@crisprtx.com Media Contact:Rachel Eides+1-617-315-4167rachel.eides@crisprtx.com
ImmunotherapyCell TherapyClinical ResultASHGene Therapy
09 Dec 2024
There were no reported dose limiting toxicities (DLTs) and no reported Grade ≥3 infections. All grade 3 or 4 cytopenias (i.e., neutropenia, thrombocytopenia, anemia) following lymphodepleting chemotherapy resolved to Grade 2 or better within 1 month of CTX112 infusion. There were no reported cases of Graft versus Host Disease (GvHD), All cases of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were Grade 1 or 2 per the American Society for Transplantation and Cellular Therapy (ASTCT) criteria. These low-grade CRS and ICANS events followed standard toxicity management protocols.
Objective and complete responses were seen at all dose levels and in all treated NHL subtypes (i.e., FL, MZL, MCL and LBCL). Responses were also seen in patients with poor prognostic factors including primary refractory disease, early relapse, and high baseline tumor burden (e.g., SPD > 4000 mm2). Five patients (of the 12 treated) have achieved responses lasting for more than 6 months, including one patient whose 6-month response was confirmed after the data cut-off date. One patient treated at DL1 remains in complete remission over a year after initial CTX112 infusion. The clinical efficacy of CTX112 is supported by a clearly differentiated pharmacokinetic profile for an allogeneic CAR T cell therapy. The mean peak concentration and total exposure were significantly higher at DL3 and DL4 vs. DL1 and DL2. This dose dependence suggests the possibility of deeper and more durable responses as the trial moves from dose escalation to dose optimization. Comparing DL3, the addition of Regnase-1 and TGFβR2 edits results in 7-fold higher peak concentration (Cmax) and 9.7-fold higher mean area under the curve (AUC) for CTX112 relative to CTX110®. Furthermore, at DL4, both Cmax and AUC are showing significantly more consistent and predictable increases. This suggests that the novel CRISPR/Cas9 potency edits are leading to higher CAR T cell expansion and functional persistence without enhanced or increased lymphodepleting chemotherapy doses.
Clinical ResultASHCell TherapyAACRImmunotherapy
05 Nov 2024
-CASGEVY™ approved for the treatment of patients 12 years of age and older with sickle cell disease (SCD) and transfusion-dependent beta thalassemia (TDT) in Switzerland and Canada-
-45 authorized treatment centers (ATCs) activated globally for CASGEVY and approximately 40 patients have had cells collected across all regions as of mid-October-
-Two clinical trials are ongoing for next generation CAR T product candidate, CTX112™ targeting CD19, in B-cell malignancies and systemic lupus erythematosus-
-Two clinical trials are ongoing for next generation CAR T product candidate, CTX131™, targeting CD70, in solid tumors and hematological malignancies-
-Company plans to provide an update on the Phase 1 dose escalation study of CTX112 in B cell malignancies at the American Society of Hematology (ASH) 2024 Annual Meeting-
-Clinical trials ongoing for in vivo gene editing product candidates, CTX310™ and CTX320™ targeting ANGPTL3 and LPA, respectively-
-Clinical trial ongoing for CTX211™, an allogeneic, hypoimmune, gene-edited, stem cell derived product candidate for the treatment of Type 1 Diabetes (T1D)-
-Strong balance sheet with approximately $1.9 billion in cash, cash equivalents, and marketable securities as of September 30, 2024-
ZUG, Switzerland and BOSTON, Nov. 05, 2024 (GLOBE NEWSWIRE) -- CRISPR Therapeutics (Nasdaq: CRSP), a biopharmaceutical company focused on creating transformative gene-based medicines for serious diseases, today reported financial results for the third quarter ended September 30, 2024.
“We continue to make significant progress across our pipeline of in vivo and ex vivo CRISPR-based therapies,” said Samarth Kulkarni, Ph.D., Chief Executive Officer and Chairman of CRISPR Therapeutics. “In addition to the continued momentum of CASGEVY’s launch, we are pleased to share that CASGEVY has received regulatory approvals for the treatment of patients 12 years of age and older with SCD or TDT in Switzerland and Canada. In parallel, we remain focused on advancing our portfolio of clinical trials across oncology, autoimmune, diabetes and cardiovascular indications in a capital efficient manner. We look forward to a number of important data catalysts over the next 9-12 months as we advance our portfolio.”
Recent Highlights and Outlook
Hemoglobinopathies and CASGEVY™ (exagamglogene autotemcel [exa-cel]) CASGEVY has received regulatory approvals for the treatment of patients 12 years of age and older with sickle cell disease (SCD) and transfusion-dependent beta thalassemia (TDT) in Switzerland and Canada. CASGEVY is also approved in the U.S., Great Britain, the European Union (EU), the Kingdom of Saudi Arabia (KSA), and the Kingdom of Bahrain (Bahrain) for the treatment of both SCD and TDT, and launches are ongoing. CASGEVY is a collaboration product between CRISPR Therapeutics and Vertex Pharmaceuticals, and as part of an amendment to the collaboration agreement in 2021, Vertex now leads global development, manufacturing, regulatory and commercialization of CASGEVY with support from CRISPR Therapeutics. As of mid-October, 45 authorized treatment centers (ATCs) have been activated globally, including centers in all regions where CASGEVY is approved, and approximately 40 patients have already had at least one cell collection across all regions. Vertex announced a reimbursement agreement with NHS England for eligible TDT patients to access CASGEVY. They have also entered into commercial discussions with NHS England to secure access to CASGEVY for eligible patients with SCD. The Italian Medicines Agency (IMA) approved the request for the implementation of an early access program (EAP), for the use of CASGEVY for the treatment of TDT and SCD. Enrollment has been completed in two global Phase 3 studies of CASGEVY in children 5 to 11 years of age with SCD or TDT and the trials are ongoing. CRISPR Therapeutics has two next generation approaches with the potential to significantly expand the addressable population with SCD and TDT. The Company continues to advance its internally developed targeted conditioning program, an anti-CD117 (c-Kit) antibody-drug conjugate (ADC), through preclinical studies. Additionally, the Company has ongoing research efforts to enable in vivo editing of hematopoietic stem cells. This work could obviate the need for conditioning altogether, expand geographic reach, and enable the treatment of multiple additional other diseases beyond SCD and TDT. Immuno-Oncology and Autoimmune Diseases CRISPR Therapeutics’ next generation allogeneic CAR T candidates reflect the Company’s mission of innovating continuously to bring potentially transformative medicines to patients as quickly as possible. Clinical trials are ongoing for the Company’s next generation CAR T product candidates, CTX112™ and CTX131™, targeting CD19 and CD70, respectively, across multiple indications. CTX112 and CTX131 both contain novel potency edits which can lead to significantly higher CAR T cell expansion and cytotoxicity, potentially representing best-in-class allogeneic CAR T products for these targets. CRISPR Therapeutics announced that it will present a poster from the Company’s ongoing Phase 1 dose escalation study evaluating the safety and efficacy of CTX112, a next-generation CD19 allogeneic CAR T cell therapy, in relapsed or refractory (r/r) CD19-positive B-cell malignancies at the American Society of Hematology (ASH) 2024 Annual Meeting. The ASH abstract includes preliminary data on nine high-risk/heavily pretreated lymphoma patients showing an overall response rate (ORR) of 67% across multiple histologies and a complete response rate (CRR) of 44%, with four patients having achieved responses lasting for more than 6 months, including one patient treated at DL1 who remains in complete remission over a year after CTX112 infusion. Concordant with efficacy, pharmacokinetic (PK) data showed rapid cell expansion for CTX112 with many fold improvements in PK compared to the previous generation CTX110® CAR T. No dose limiting toxicities (DLTs) or adverse events (AEs) of graft versus host disease, hemophagocytic lymphohistiocytosis, or grade (Gr) ≥3 infections were observed, in addition to no Gr ≥3 cytokine release syndrome (CRS), or Gr ≥2 immune effector cell associated neurotoxicity syndrome (ICANS). Compared with first generation allogeneic CAR T therapies like CTX110, CTX112 results in better efficacy at lower doses, higher response rates, and improved PK. These data provide the first clinical evidence that disruptions in the genes encoding Regnase-1 and transforming growth factor beta receptor 2 can lead to increased expansion and functional persistence of CAR T cells. The poster presentation will include additional results from the trial. CTX112 is also in a Phase 1 clinical trial in systemic lupus erythematosus (SLE), with the potential to expand into additional autoimmune indications in the future. Early clinical studies conducted by third parties have shown that CD19-directed autologous CAR T therapy can produce long-lasting remissions in multiple autoimmune indications by deeply depleting B cells. The Company’s first generation allogeneic CD19-directed CAR T program has demonstrated effective depletion of B cells in oncology settings, which supports the potential for CTX112 in autoimmune diseases. CTX131 is currently in ongoing clinical trials in solid tumors and hematologic malignancies including T cell lymphomas (TCL). The Company plans to announce an update from the Phase 1 solid tumor trial in 2025. In certain hematologic malignancies such as TCL, allogeneic CAR T approaches may have greater potential to meet the unmet need in this patient population given the patients’ own T cells are not suitable for autologous manufacturing. In Vivo CRISPR Therapeutics has established a proprietary lipid nanoparticle (LNP) platform for the delivery of CRISPR/Cas9 to the liver. The first two in vivo programs utilizing this proprietary platform, CTX310™ and CTX320™, are directed towards validated therapeutic targets associated with cardiovascular disease. CTX310 is currently in an ongoing Phase 1 clinical trial targeting ANGPTL3 in patients with homozygous familial hypercholesterolemia (HoFH), severe hypertriglyceridemia (SHTG), heterozygous familial hypercholesterolemia (HeFH), or mixed dyslipidemias. Natural loss-of-function mutations in ANGPTL3 are associated with reduced low-density lipoprotein (LDL-C), triglycerides (TG) and atherosclerotic cardiovascular disease (ASCVD) risk without any negative impact on overall health. CRISPR Therapeutics expects to provide an update from this program in 2025. CTX320 is currently in an ongoing Phase 1 clinical trial targeting LPA in patients with elevated lipoprotein(a) [Lp(a)], which has shown to have an independent association with major adverse cardiovascular events (MACE). Up to 20% of the global population has elevated Lp(a) levels. CRISPR Therapeutics expects to provide an update from this program in 2025. The Company continues to advance two additional preclinical programs, CTX340™ targeting angiotensinogen (AGT) for the treatment of refractory hypertension and CTX450™ targeting 5’ aminolevulinic acid synthase 1 (ALAS1) for the treatment of acute hepatic porphyrias (AHP). CRISPR Therapeutics is conducting IND/CTA-enabling studies and expects to initiate both clinical trials in the second half of 2025. Regenerative Medicine CTX211™, an allogeneic, gene-edited, stem cell-derived beta islet cell precursor, is currently in an ongoing Phase 1 clinical trial for the treatment of Type 1 Diabetes (T1D). CRISPR Therapeutics remains committed to its goal of developing a beta-cell replacement product that does not require chronic immunosuppression. Vertex has non-exclusive rights to certain CRISPR Therapeutics’ CRISPR/Cas9 technology to accelerate development of potentially curative cell therapies for T1D. CRISPR Therapeutics remains eligible for development milestones and would receive royalties on any future products resulting from this agreement. Third Quarter 2024 Financial Results Cash Position: Cash, cash equivalents, and marketable securities were $1,935.6 million as of September 30, 2024, compared to $1,695.7 million as of December 31, 2023. The increase in cash was primarily driven by proceeds from the $280.0 million February 2024 registered direct offering, a $200.0 million milestone payment received from Vertex Pharmaceuticals in connection with the approval of CASGEVY, proceeds from employee option exercises as well as interest income, offset by operating expenses. R&D Expenses: R&D expenses were $82.2 million for the third quarter of 2024, compared to $90.7 million for the third quarter of 2023. The decrease in R&D expense was primarily driven by reduced variable external research and manufacturing costs. G&A Expenses: General and administrative expenses were $17.4 million for the third quarter of 2024, compared to $18.3 million for the third quarter of 2023. Collaboration Expense: Collaboration expense, net, was $11.2 million for the third quarter of 2024, compared to $23.4 million for the third quarter of 2023. The decrease in collaboration expense, net, was primarily attributable to the time of reaching the deferral limit on costs related to the CASGEVY program. Net Loss: Net loss was $85.9 million for the third quarter of 2024, compared to a net loss of $112.2 million for the third quarter of 2023.
Hemoglobinopathies and CASGEVY™ (exagamglogene autotemcel [exa-cel])
CASGEVY has received regulatory approvals for the treatment of patients 12 years of age and older with sickle cell disease (SCD) and transfusion-dependent beta thalassemia (TDT) in Switzerland and Canada. CASGEVY is also approved in the U.S., Great Britain, the European Union (EU), the Kingdom of Saudi Arabia (KSA), and the Kingdom of Bahrain (Bahrain) for the treatment of both SCD and TDT, and launches are ongoing. CASGEVY is a collaboration product between CRISPR Therapeutics and Vertex Pharmaceuticals, and as part of an amendment to the collaboration agreement in 2021, Vertex now leads global development, manufacturing, regulatory and commercialization of CASGEVY with support from CRISPR Therapeutics. As of mid-October, 45 authorized treatment centers (ATCs) have been activated globally, including centers in all regions where CASGEVY is approved, and approximately 40 patients have already had at least one cell collection across all regions. Vertex announced a reimbursement agreement with NHS England for eligible TDT patients to access CASGEVY. They have also entered into commercial discussions with NHS England to secure access to CASGEVY for eligible patients with SCD. The Italian Medicines Agency (IMA) approved the request for the implementation of an early access program (EAP), for the use of CASGEVY for the treatment of TDT and SCD. Enrollment has been completed in two global Phase 3 studies of CASGEVY in children 5 to 11 years of age with SCD or TDT and the trials are ongoing. CRISPR Therapeutics has two next generation approaches with the potential to significantly expand the addressable population with SCD and TDT. The Company continues to advance its internally developed targeted conditioning program, an anti-CD117 (c-Kit) antibody-drug conjugate (ADC), through preclinical studies. Additionally, the Company has ongoing research efforts to enable in vivo editing of hematopoietic stem cells. This work could obviate the need for conditioning altogether, expand geographic reach, and enable the treatment of multiple additional other diseases beyond SCD and TDT.
CASGEVY has received regulatory approvals for the treatment of patients 12 years of age and older with sickle cell disease (SCD) and transfusion-dependent beta thalassemia (TDT) in Switzerland and Canada. CASGEVY is also approved in the U.S., Great Britain, the European Union (EU), the Kingdom of Saudi Arabia (KSA), and the Kingdom of Bahrain (Bahrain) for the treatment of both SCD and TDT, and launches are ongoing. CASGEVY is a collaboration product between CRISPR Therapeutics and Vertex Pharmaceuticals, and as part of an amendment to the collaboration agreement in 2021, Vertex now leads global development, manufacturing, regulatory and commercialization of CASGEVY with support from CRISPR Therapeutics.
As of mid-October, 45 authorized treatment centers (ATCs) have been activated globally, including centers in all regions where CASGEVY is approved, and approximately 40 patients have already had at least one cell collection across all regions.
Vertex announced a reimbursement agreement with NHS England for eligible TDT patients to access CASGEVY. They have also entered into commercial discussions with NHS England to secure access to CASGEVY for eligible patients with SCD.
The Italian Medicines Agency (IMA) approved the request for the implementation of an early access program (EAP), for the use of CASGEVY for the treatment of TDT and SCD.
Enrollment has been completed in two global Phase 3 studies of CASGEVY in children 5 to 11 years of age with SCD or TDT and the trials are ongoing.
CRISPR Therapeutics has two next generation approaches with the potential to significantly expand the addressable population with SCD and TDT. The Company continues to advance its internally developed targeted conditioning program, an anti-CD117 (c-Kit) antibody-drug conjugate (ADC), through preclinical studies. Additionally, the Company has ongoing research efforts to enable in vivo editing of hematopoietic stem cells. This work could obviate the need for conditioning altogether, expand geographic reach, and enable the treatment of multiple additional other diseases beyond SCD and TDT.
Immuno-Oncology and Autoimmune Diseases
CRISPR Therapeutics’ next generation allogeneic CAR T candidates reflect the Company’s mission of innovating continuously to bring potentially transformative medicines to patients as quickly as possible. Clinical trials are ongoing for the Company’s next generation CAR T product candidates, CTX112™ and CTX131™, targeting CD19 and CD70, respectively, across multiple indications. CTX112 and CTX131 both contain novel potency edits which can lead to significantly higher CAR T cell expansion and cytotoxicity, potentially representing best-in-class allogeneic CAR T products for these targets. CRISPR Therapeutics announced that it will present a poster from the Company’s ongoing Phase 1 dose escalation study evaluating the safety and efficacy of CTX112, a next-generation CD19 allogeneic CAR T cell therapy, in relapsed or refractory (r/r) CD19-positive B-cell malignancies at the American Society of Hematology (ASH) 2024 Annual Meeting. The ASH abstract includes preliminary data on nine high-risk/heavily pretreated lymphoma patients showing an overall response rate (ORR) of 67% across multiple histologies and a complete response rate (CRR) of 44%, with four patients having achieved responses lasting for more than 6 months, including one patient treated at DL1 who remains in complete remission over a year after CTX112 infusion. Concordant with efficacy, pharmacokinetic (PK) data showed rapid cell expansion for CTX112 with many fold improvements in PK compared to the previous generation CTX110® CAR T. No dose limiting toxicities (DLTs) or adverse events (AEs) of graft versus host disease, hemophagocytic lymphohistiocytosis, or grade (Gr) ≥3 infections were observed, in addition to no Gr ≥3 cytokine release syndrome (CRS), or Gr ≥2 immune effector cell associated neurotoxicity syndrome (ICANS). Compared with first generation allogeneic CAR T therapies like CTX110, CTX112 results in better efficacy at lower doses, higher response rates, and improved PK. These data provide the first clinical evidence that disruptions in the genes encoding Regnase-1 and transforming growth factor beta receptor 2 can lead to increased expansion and functional persistence of CAR T cells. The poster presentation will include additional results from the trial. CTX112 is also in a Phase 1 clinical trial in systemic lupus erythematosus (SLE), with the potential to expand into additional autoimmune indications in the future. Early clinical studies conducted by third parties have shown that CD19-directed autologous CAR T therapy can produce long-lasting remissions in multiple autoimmune indications by deeply depleting B cells. The Company’s first generation allogeneic CD19-directed CAR T program has demonstrated effective depletion of B cells in oncology settings, which supports the potential for CTX112 in autoimmune diseases. CTX131 is currently in ongoing clinical trials in solid tumors and hematologic malignancies including T cell lymphomas (TCL). The Company plans to announce an update from the Phase 1 solid tumor trial in 2025. In certain hematologic malignancies such as TCL, allogeneic CAR T approaches may have greater potential to meet the unmet need in this patient population given the patients’ own T cells are not suitable for autologous manufacturing.
CRISPR Therapeutics’ next generation allogeneic CAR T candidates reflect the Company’s mission of innovating continuously to bring potentially transformative medicines to patients as quickly as possible. Clinical trials are ongoing for the Company’s next generation CAR T product candidates, CTX112™ and CTX131™, targeting CD19 and CD70, respectively, across multiple indications. CTX112 and CTX131 both contain novel potency edits which can lead to significantly higher CAR T cell expansion and cytotoxicity, potentially representing best-in-class allogeneic CAR T products for these targets.
CRISPR Therapeutics announced that it will present a poster from the Company’s ongoing Phase 1 dose escalation study evaluating the safety and efficacy of CTX112, a next-generation CD19 allogeneic CAR T cell therapy, in relapsed or refractory (r/r) CD19-positive B-cell malignancies at the American Society of Hematology (ASH) 2024 Annual Meeting. The ASH abstract includes preliminary data on nine high-risk/heavily pretreated lymphoma patients showing an overall response rate (ORR) of 67% across multiple histologies and a complete response rate (CRR) of 44%, with four patients having achieved responses lasting for more than 6 months, including one patient treated at DL1 who remains in complete remission over a year after CTX112 infusion. Concordant with efficacy, pharmacokinetic (PK) data showed rapid cell expansion for CTX112 with many fold improvements in PK compared to the previous generation CTX110® CAR T. No dose limiting toxicities (DLTs) or adverse events (AEs) of graft versus host disease, hemophagocytic lymphohistiocytosis, or grade (Gr) ≥3 infections were observed, in addition to no Gr ≥3 cytokine release syndrome (CRS), or Gr ≥2 immune effector cell associated neurotoxicity syndrome (ICANS). Compared with first generation allogeneic CAR T therapies like CTX110, CTX112 results in better efficacy at lower doses, higher response rates, and improved PK. These data provide the first clinical evidence that disruptions in the genes encoding Regnase-1 and transforming growth factor beta receptor 2 can lead to increased expansion and functional persistence of CAR T cells. The poster presentation will include additional results from the trial.
CTX112 is also in a Phase 1 clinical trial in systemic lupus erythematosus (SLE), with the potential to expand into additional autoimmune indications in the future. Early clinical studies conducted by third parties have shown that CD19-directed autologous CAR T therapy can produce long-lasting remissions in multiple autoimmune indications by deeply depleting B cells. The Company’s first generation allogeneic CD19-directed CAR T program has demonstrated effective depletion of B cells in oncology settings, which supports the potential for CTX112 in autoimmune diseases.
CTX131 is currently in ongoing clinical trials in solid tumors and hematologic malignancies including T cell lymphomas (TCL). The Company plans to announce an update from the Phase 1 solid tumor trial in 2025. In certain hematologic malignancies such as TCL, allogeneic CAR T approaches may have greater potential to meet the unmet need in this patient population given the patients’ own T cells are not suitable for autologous manufacturing.
In Vivo
CRISPR Therapeutics has established a proprietary lipid nanoparticle (LNP) platform for the delivery of CRISPR/Cas9 to the liver. The first two in vivo programs utilizing this proprietary platform, CTX310™ and CTX320™, are directed towards validated therapeutic targets associated with cardiovascular disease. CTX310 is currently in an ongoing Phase 1 clinical trial targeting ANGPTL3 in patients with homozygous familial hypercholesterolemia (HoFH), severe hypertriglyceridemia (SHTG), heterozygous familial hypercholesterolemia (HeFH), or mixed dyslipidemias. Natural loss-of-function mutations in ANGPTL3 are associated with reduced low-density lipoprotein (LDL-C), triglycerides (TG) and atherosclerotic cardiovascular disease (ASCVD) risk without any negative impact on overall health. CRISPR Therapeutics expects to provide an update from this program in 2025. CTX320 is currently in an ongoing Phase 1 clinical trial targeting LPA in patients with elevated lipoprotein(a) [Lp(a)], which has shown to have an independent association with major adverse cardiovascular events (MACE). Up to 20% of the global population has elevated Lp(a) levels. CRISPR Therapeutics expects to provide an update from this program in 2025. The Company continues to advance two additional preclinical programs, CTX340™ targeting angiotensinogen (AGT) for the treatment of refractory hypertension and CTX450™ targeting 5’ aminolevulinic acid synthase 1 (ALAS1) for the treatment of acute hepatic porphyrias (AHP). CRISPR Therapeutics is conducting IND/CTA-enabling studies and expects to initiate both clinical trials in the second half of 2025.
CRISPR Therapeutics has established a proprietary lipid nanoparticle (LNP) platform for the delivery of CRISPR/Cas9 to the liver. The first two in vivo programs utilizing this proprietary platform, CTX310™ and CTX320™, are directed towards validated therapeutic targets associated with cardiovascular disease.
CTX310 is currently in an ongoing Phase 1 clinical trial targeting ANGPTL3 in patients with homozygous familial hypercholesterolemia (HoFH), severe hypertriglyceridemia (SHTG), heterozygous familial hypercholesterolemia (HeFH), or mixed dyslipidemias. Natural loss-of-function mutations in ANGPTL3 are associated with reduced low-density lipoprotein (LDL-C), triglycerides (TG) and atherosclerotic cardiovascular disease (ASCVD) risk without any negative impact on overall health. CRISPR Therapeutics expects to provide an update from this program in 2025.
CTX320 is currently in an ongoing Phase 1 clinical trial targeting LPA in patients with elevated lipoprotein(a) [Lp(a)], which has shown to have an independent association with major adverse cardiovascular events (MACE). Up to 20% of the global population has elevated Lp(a) levels. CRISPR Therapeutics expects to provide an update from this program in 2025.
The Company continues to advance two additional preclinical programs, CTX340™ targeting angiotensinogen (AGT) for the treatment of refractory hypertension and CTX450™ targeting 5’ aminolevulinic acid synthase 1 (ALAS1) for the treatment of acute hepatic porphyrias (AHP). CRISPR Therapeutics is conducting IND/CTA-enabling studies and expects to initiate both clinical trials in the second half of 2025.
Regenerative Medicine
CTX211™, an allogeneic, gene-edited, stem cell-derived beta islet cell precursor, is currently in an ongoing Phase 1 clinical trial for the treatment of Type 1 Diabetes (T1D). CRISPR Therapeutics remains committed to its goal of developing a beta-cell replacement product that does not require chronic immunosuppression. Vertex has non-exclusive rights to certain CRISPR Therapeutics’ CRISPR/Cas9 technology to accelerate development of potentially curative cell therapies for T1D. CRISPR Therapeutics remains eligible for development milestones and would receive royalties on any future products resulting from this agreement.
CTX211™, an allogeneic, gene-edited, stem cell-derived beta islet cell precursor, is currently in an ongoing Phase 1 clinical trial for the treatment of Type 1 Diabetes (T1D). CRISPR Therapeutics remains committed to its goal of developing a beta-cell replacement product that does not require chronic immunosuppression.
Vertex has non-exclusive rights to certain CRISPR Therapeutics’ CRISPR/Cas9 technology to accelerate development of potentially curative cell therapies for T1D. CRISPR Therapeutics remains eligible for development milestones and would receive royalties on any future products resulting from this agreement.
Third Quarter 2024 Financial Results
Cash Position: Cash, cash equivalents, and marketable securities were $1,935.6 million as of September 30, 2024, compared to $1,695.7 million as of December 31, 2023. The increase in cash was primarily driven by proceeds from the $280.0 million February 2024 registered direct offering, a $200.0 million milestone payment received from Vertex Pharmaceuticals in connection with the approval of CASGEVY, proceeds from employee option exercises as well as interest income, offset by operating expenses. R&D Expenses: R&D expenses were $82.2 million for the third quarter of 2024, compared to $90.7 million for the third quarter of 2023. The decrease in R&D expense was primarily driven by reduced variable external research and manufacturing costs. G&A Expenses: General and administrative expenses were $17.4 million for the third quarter of 2024, compared to $18.3 million for the third quarter of 2023. Collaboration Expense: Collaboration expense, net, was $11.2 million for the third quarter of 2024, compared to $23.4 million for the third quarter of 2023. The decrease in collaboration expense, net, was primarily attributable to the time of reaching the deferral limit on costs related to the CASGEVY program. Net Loss: Net loss was $85.9 million for the third quarter of 2024, compared to a net loss of $112.2 million for the third quarter of 2023.
Cash Position: Cash, cash equivalents, and marketable securities were $1,935.6 million as of September 30, 2024, compared to $1,695.7 million as of December 31, 2023. The increase in cash was primarily driven by proceeds from the $280.0 million February 2024 registered direct offering, a $200.0 million milestone payment received from Vertex Pharmaceuticals in connection with the approval of CASGEVY, proceeds from employee option exercises as well as interest income, offset by operating expenses.
R&D Expenses: R&D expenses were $82.2 million for the third quarter of 2024, compared to $90.7 million for the third quarter of 2023. The decrease in R&D expense was primarily driven by reduced variable external research and manufacturing costs.
G&A Expenses: General and administrative expenses were $17.4 million for the third quarter of 2024, compared to $18.3 million for the third quarter of 2023.
Collaboration Expense: Collaboration expense, net, was $11.2 million for the third quarter of 2024, compared to $23.4 million for the third quarter of 2023. The decrease in collaboration expense, net, was primarily attributable to the time of reaching the deferral limit on costs related to the CASGEVY program.
Net Loss: Net loss was $85.9 million for the third quarter of 2024, compared to a net loss of $112.2 million for the third quarter of 2023.
About CASGEVY (exagamglogene autotemcel [exa-cel]) CASGEVY is a non-viral, ex vivo CRISPR/Cas9 gene-edited cell therapy for eligible patients with SCD or TDT, in which a patient’s own hematopoietic stem and progenitor cells are edited at the erythroid specific enhancer region of the BCL11A gene. This edit results in the production of high levels of fetal hemoglobin (HbF; hemoglobin F) in red blood cells. HbF is the form of the oxygen-carrying hemoglobin that is naturally present during fetal development, which then switches to the adult form of hemoglobin after birth. CASGEVY has been shown to reduce or eliminate VOCs for patients with SCD and transfusion requirements for patients with TDT. CASGEVY is approved for certain indications in multiple jurisdictions for eligible patients.
About the CRISPR Therapeutics-Vertex Collaboration CRISPR Therapeutics and Vertex entered into a strategic research collaboration in 2015 focused on the use of CRISPR/Cas9 to discover and develop potential new treatments aimed at the underlying genetic causes of human disease. CASGEVY represents the first potential treatment to emerge from the joint research program. Under an amended collaboration agreement, Vertex now leads global development, manufacturing, and commercialization of CASGEVY and splits program costs and profits worldwide 60/40 with CRISPR Therapeutics. Vertex is the manufacturer and exclusive license holder of CASGEVY.
About CTX112 CTX112 is being developed for both oncology and autoimmune indications. CTX112 is a next-generation, wholly-owned, allogeneic CAR T product candidate targeting Cluster of Differentiation 19, or CD19, which incorporates edits designed to evade the immune system, enhance CAR T potency and reduce CAR T exhaustion. CTX112 is being investigated in an ongoing clinical trial designed to assess safety and efficacy of the product candidate in adult patients with relapsed or refractory CD19-positive B-cell malignancies who have received at least two prior lines of therapy. In addition, CTX112 is being investigated in an ongoing clinical trial designed to assess safety and efficacy of the product candidate in adult patients with system lupus erythematosus.
About CTX131 CTX131 is being developed for both solid tumors and hematologic malignancies, including T cell lymphomas (TCL). CTX131 is a next-generation, wholly-owned, allogeneic CAR T product candidate targeting Cluster of Differentiation 70, or CD70, an antigen expressed on various solid tumors and hematologic malignancies. CTX131 incorporates edits designed to evade the immune system, prevent fratricide, enhance CAR T potency and reduce CAR T exhaustion. CTX131 is being investigated in ongoing clinical trials designed to assess the safety and efficacy of the product candidate in adult patients with relapsed or refractory solid tumors and hematologic malignancies, including TCL.
About In Vivo Programs CRISPR Therapeutics has established a proprietary lipid nanoparticle (LNP) platform for the delivery of CRISPR/Cas9 to the liver. The Company’s in vivo portfolio includes its lead investigational programs, CTX310 (directed towards angiopoietin-related protein 3 (ANGPTL3)) and CTX320 (directed towards LPA, the gene encoding apolipoprotein(a) (apo(a)), a major component of lipoprotein(a) [Lp(a)]). Both are targeting validated therapeutic targets for cardiovascular disease. CTX310 and CTX320 are in ongoing clinical trials in patients with heterozygous familial hypercholesterolemia, homozygous familial hypercholesterolemia, mixed dyslipidemias, or severe hypertriglyceridemia, and in patients with elevated lipoprotein(a), respectively. In addition, the Company’s research and preclinical development candidates include CTX340 and CTX450, targeting angiotensinogen (AGT) for refractory hypertension and 5’-aminolevulinate synthase 1 (ALAS1) for acute hepatic porphyria (AHP), respectively.
About CTX211 CTX211 is an allogeneic, gene-edited, stem cell-derived investigational therapy for the treatment of type 1 diabetes (T1D), which incorporates gene edits that aim to make cells hypoimmune and enhance cell fitness. This immune-evasive cell replacement therapy is designed to enable patients to produce their own insulin in response to glucose. A Phase 1 clinical trial for CTX211 for the treatment of T1D is ongoing.
About CRISPR Therapeutics Since its inception over a decade ago, CRISPR Therapeutics has transformed from a research-stage company advancing programs in the field of gene editing, to a company that recently celebrated the historic approval of the first-ever CRISPR-based therapy and has a diverse portfolio of product candidates across a broad range of disease areas including hemoglobinopathies, oncology, regenerative medicine, cardiovascular, autoimmune, and rare diseases. CRISPR Therapeutics advanced the first-ever CRISPR/Cas9 gene-edited therapy into the clinic in 2018 to investigate the treatment of sickle cell disease or transfusion-dependent beta thalassemia, and beginning in late 2023, CASGEVY (exagamglogene autotemcel [exa-cel]) was approved in some countries to treat eligible patients with either of those conditions. The Nobel Prize-winning CRISPR science has revolutionized biomedical research and represents a powerful, clinically validated approach with the potential to create a new class of potentially transformative medicines. To accelerate and expand its efforts, CRISPR Therapeutics has established strategic partnerships with leading companies including Bayer and Vertex Pharmaceuticals. CRISPR Therapeutics AG is headquartered in Zug, Switzerland, with its wholly-owned U.S. subsidiary, CRISPR Therapeutics, Inc., and R&D operations based in Boston, Massachusetts and San Francisco, California, and business offices in London, United Kingdom. To learn more, visit www.crisprtx.com.
CRISPR THERAPEUTICS® standard character mark and design logo, CTX110®, CTX112™, CTX131™, CTX211™, CTX310™, CTX320™, CTX340™ and CTX450™ are trademarks and registered trademarks of CRISPR Therapeutics AG. The CASGEVY™ word mark and design are trademarks of Vertex Pharmaceuticals Incorporated. All other trademarks and registered trademarks are the property of their respective owners.
CRISPR Therapeutics Forward-Looking Statement Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding any or all of the following: (i) CRISPR Therapeutics preclinical studies, clinical trials and pipeline products and programs, including, without limitation, manufacturing capabilities, status of such studies and trials, potential expansion into new indications and expectations regarding data, safety and efficacy generally; as well as its plans to and the clinical data being presented at the 2024 ASH Annual Meeting; (ii) its strategy, goals, anticipated financial performance and the sufficiency of its cash resources; (iii) regulatory submissions and authorizations, including timelines for and expectations regarding additional regulatory agency decisions; (iv) the expected benefits of its collaborations; and (v) the therapeutic value, development, and commercial potential of gene editing technologies and therapies, including CRISPR/Cas9. Risks that contribute to the uncertain nature of the forward-looking statements include, without limitation, the risks and uncertainties discussed under the heading “Risk Factors” in its most recent annual report on Form 10-K and in any other subsequent filings made by CRISPR Therapeutics with the U.S. Securities and Exchange Commission. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date they are made. We disclaim any obligation or undertaking to update or revise any forward-looking statements contained in this press release, other than to the extent required by law.
This press release discusses CRISPR/Cas9 gene editing investigational therapies and is not intended to convey conclusions about efficacy or safety as to those investigational therapies or uses of such investigational therapies. There is no guarantee that any investigational therapy will successfully complete clinical development or gain approval from applicable regulatory authorities.
Investor Contact: Susie Kim +1-617-307-7503 susan.kim@crisprtx.com
Media Contact: Rachel Eides +1-617-315-4493 rachel.eides@crisprtx.com
Condensed Consolidated Statements of Operations (Unaudited, In thousands except share data and per share data)
Condensed Consolidated Balance Sheets Data (Unaudited, in thousands)
Source: CRISPR Therapeutics AG
Phase 1Drug ApprovalPhase 3Cell TherapyLicense out/in
100 Deals associated with evoncabtagene pazurgedleucel
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Adult Acute Lymphocytic Leukemia | Phase 2 | United States | 22 Jul 2019 | |
| Adult Acute Lymphocytic Leukemia | Phase 2 | Australia | 22 Jul 2019 | |
| Adult Acute Lymphocytic Leukemia | Phase 2 | Canada | 22 Jul 2019 | |
| Adult Acute Lymphocytic Leukemia | Phase 2 | France | 22 Jul 2019 | |
| Adult Acute Lymphocytic Leukemia | Phase 2 | Germany | 22 Jul 2019 | |
| Adult Acute Lymphocytic Leukemia | Phase 2 | Spain | 22 Jul 2019 | |
| B-Cell Lymphoma | Phase 2 | United States | 22 Jul 2019 | |
| B-Cell Lymphoma | Phase 2 | Australia | 22 Jul 2019 | |
| B-Cell Lymphoma | Phase 2 | Canada | 22 Jul 2019 | |
| B-Cell Lymphoma | Phase 2 | France | 22 Jul 2019 |
Login to view more data
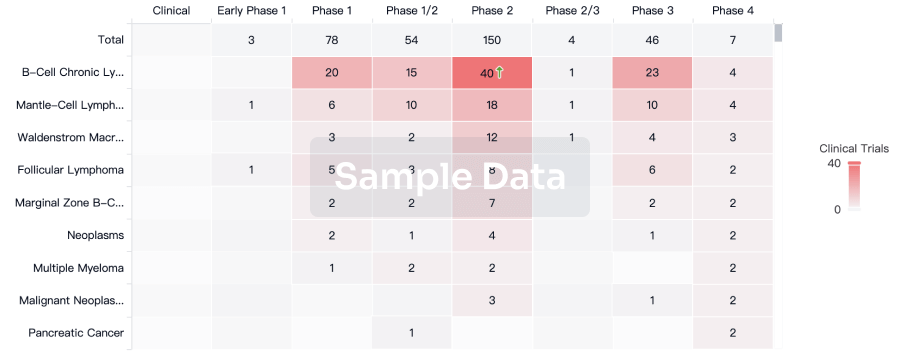
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 1 | 32 | fludarabine+cyclophosphamide+CTX110 | pslyipaysm(irtuomakna) = vlsyuprkye tzsmottqcv (rguwoznebd ) View more | Positive | 15 Nov 2022 |
Login to view more data
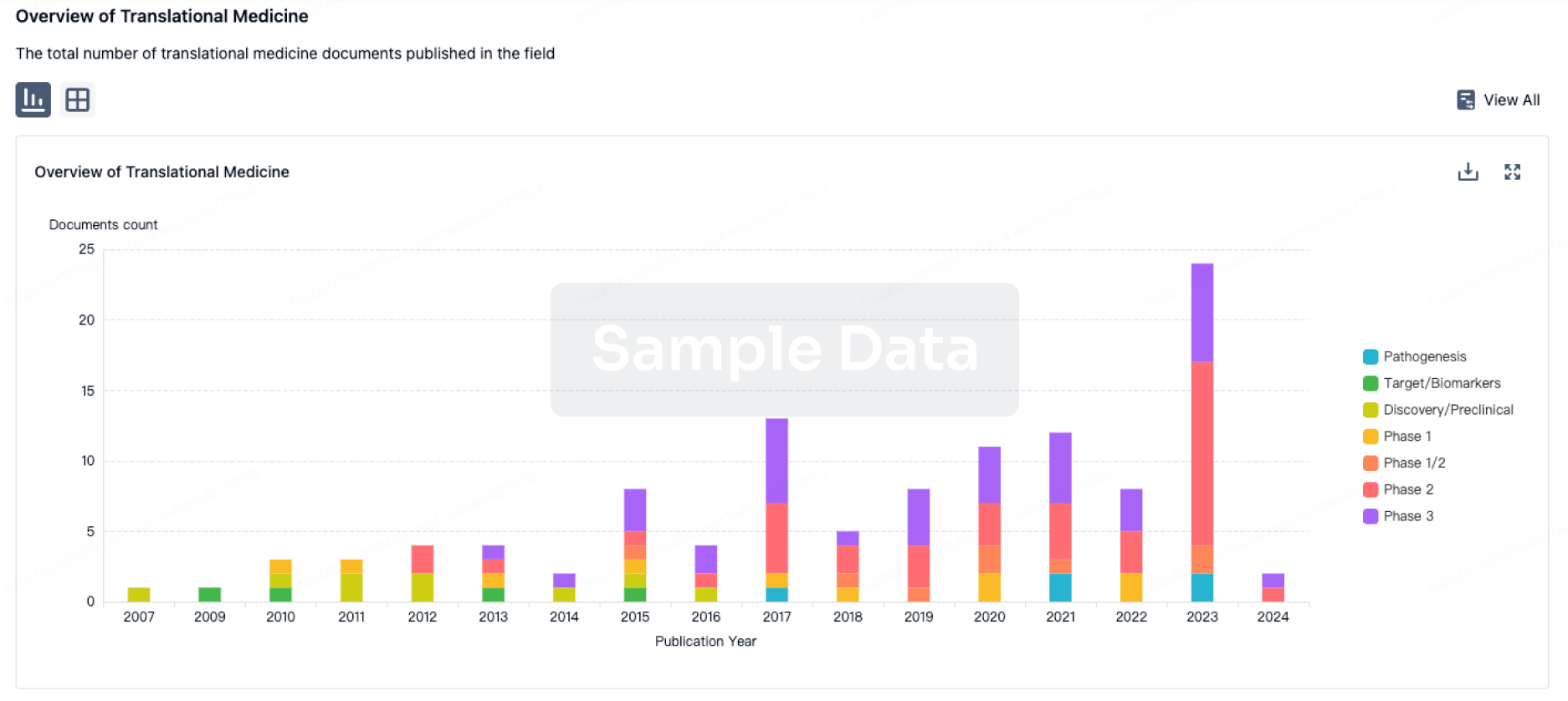
Translational Medicine
Boost your research with our translational medicine data.
login
or
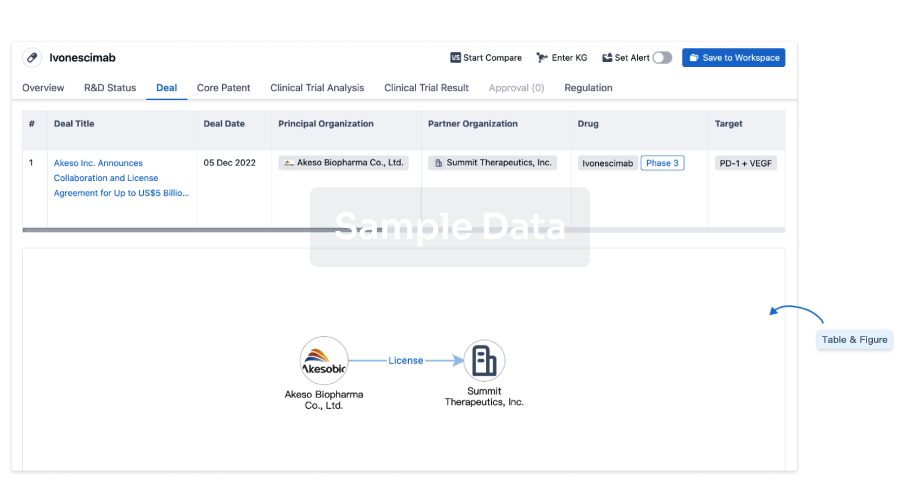
Deal
Boost your decision using our deal data.
login
or
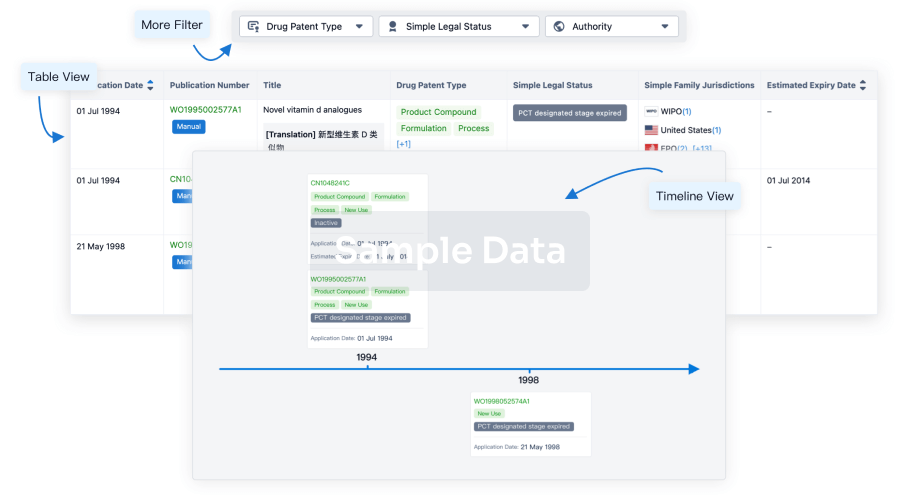
Core Patent
Boost your research with our Core Patent data.
login
or
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
