Request Demo
Last update 20 Dec 2025
Rosiptor
Last update 20 Dec 2025
Overview
Basic Info
Drug Type Small molecule drug |
Synonyms Rosiptor (USAN/INN), Rosiptor acetate, AQX 1125 + [1] |
Target |
Action activators |
Mechanism SHIP1 activators(Phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase 1 activators) |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
License Organization |
Drug Highest PhaseDiscontinuedPhase 3 |
First Approval Date- |
Regulation- |
Login to view timeline
Structure/Sequence
Molecular FormulaC22H39NO4 |
InChIKeyCQIBMEIJDDUKHP-BAHZVNHDSA-N |
CAS Registry782487-29-0 |
Related
8
Clinical Trials associated with RosiptorNCT03500159
A 12-Week, Randomized, Multi-Center, Double-Blind, Placebo-Controlled, Parallel-Group, Phase 2 Trial to Evaluate the Efficacy and Safety of AQX-1125 (200 mg) in Male Subjects With Chronic Prostatitis/Chronic Pelvic Pain Syndrome
This is a randomized, multi-center, double-blind, parallel-group study, enrolling approximately 100 male subjects diagnosed with CP/CPPS to evaluate the effect of 12-week treatment with AQX-1125 (active drug) compared to placebo.
The subjects will be randomized to receive orally once-daily either AQX-1125 (200 mg) or placebo in a 1:1 ratio across approximately 30 centers in North America (United States and Canada). The study will consist of a screening period of up to 3 weeks, a 12-week treatment period followed by a 4-week off drug safety follow-up period, and an ophthalmic safety follow-up call at 3 months and visit at 6 months post last dose, for a total study duration of about 41 weeks.
The subjects will be randomized to receive orally once-daily either AQX-1125 (200 mg) or placebo in a 1:1 ratio across approximately 30 centers in North America (United States and Canada). The study will consist of a screening period of up to 3 weeks, a 12-week treatment period followed by a 4-week off drug safety follow-up period, and an ophthalmic safety follow-up call at 3 months and visit at 6 months post last dose, for a total study duration of about 41 weeks.
Start Date18 Apr 2018 |
Sponsor / Collaborator |
NCT03478020
Open-Label, Fixed-Sequence 3-Period Study to Determine the Effects of Repeated Oral Dosing of AQX-1125 on the Pharamacokinetics, Safety and Tolerability of a Combination Oral Contraceptive in Healthy Female Subjects
This is an open-label, fixed sequence, 4 cycle, drug-drug interaction (DDI) study of AQX-1125 in healthy female subjects on combination oral contraceptives (COC).
Start Date22 Nov 2017 |
Sponsor / Collaborator |
NCT03185195
An Open-Label,2-Part Sequential Dose Study Designed to Assess the Absolute Bioavailability, Mass Balance Recovery, Metabolite Profile and Identification of Metabolite Structure for [14C]-AQX-1125 in Healthy Subjects
This study is conducted to evaluate the absolute bioavailability, metabolism and elimination pathways of AQX-1125 in healthy male and female subjects.
Start Date22 Nov 2016 |
Sponsor / Collaborator |
100 Clinical Results associated with Rosiptor
Login to view more data
100 Translational Medicine associated with Rosiptor
Login to view more data
100 Patents (Medical) associated with Rosiptor
Login to view more data
11
Literatures (Medical) associated with Rosiptor01 Nov 2022·Antiviral research
SHIP-1 affects herpetic simplex keratitis prognosis by mediating CD4+ T lymphocytes migration through PI3K signaling and transcription factor KLF2 in the cornea
Article
Author: Qin, Qiyu ; Jin, Xiuming ; Huang, Xiaodan ; Wang, Yi
Herpetic simplex keratitis (HSK) mainly represents an immune cell-mediated, and more specifically, CD4+ T cell-orchestrated inflammatory response to virus invasion. The virus in infected corneas could be easily inhibited or hidden in the trigeminal ganglion using antiviral drugs, but the immune-related inflammation will last for a long time and lead to significant complications. In the present study, we found that the subconjunctival injection of SHIP-1 activator AQX1125 in mouse HSK model alleviated the corneal inflammatory and angiogenic responses, as well as promoted quicker recovery of the cornea, with significantly fewer infiltration of CD4+ T lymphocytes. Furthermore, using primary CD4+ T lymphocytes, we observed that by modulating PI3K signaling and the expression of transcription factors KLF2 and CCR7, SHIP-1 could significantly influence the migration of lymphocytes toward CCL19 and 21, which are the "exit cues" for cells to emigrate from inflammatory sites. Thus, we propose that the pharmacological SHIP-1 activation represents a new potential therapeutic approach to control HSK lesions, and its function on the CCR7-CCL19/21 biological axis may be a novel underlying mechanism for its anti-inflammatory action.
01 Jan 2018·European urology focusQ2 · MEDICINE
Lower Urinary Tract Symptoms: What’s New in Medical Treatment?
Q2 · MEDICINE
Review
Author: Brucker, Benjamin M ; Michel, Martin C ; Peyronnet, Benoit
CONTEXT:
Pharmacological treatment is a cornerstone in the management of patients with lower urinary tract symptoms (LUTS).
OBJECTIVE:
To review emerging evidence in the medical treatment of LUTS.
EVIDENCE ACQUISITION:
An Embase/Pubmed-based literature search was conducted in December 2017, screening for randomized controlled trials (RCTs), prospective and retrospective series, animal model studies, and reviews on medical treatment of LUTS.
EVIDENCE SYNTHESIS:
The main medical innovation in recent years in overactive bladder (OAB) has been the approval of the first β3-adrenoceptor agonists (mirabegron) and intradetrusor onabotulinum toxin A, while several other drugs such as antiepileptics, phosphodiesterase inhibitors, or other β3-agonists have brought promising results in phase 3 trials. Intraprostatic injections of various drugs for LUTS/benign prostatic hyperplasia have been investigated, but results of phase 3 trials are still pending, while combination therapies of phosphodiesterase type 5 inhibitors+α-blockers or finasteride have been proved as superior to single therapies in RCTs conducted in these patients. Two new formulations of desmopressin have been approved for nocturia in the USA (desmopressin nasal spray) and Europe/Canada/Australia (desmopressin orally disintegrated tablet). Fedovapagon, a vasopressin V2 receptor agonist, has recently completed a large phase 3 trial in male patients with nocturia. Other phase 3 trials are ongoing in bladder pain syndrome (AQX 11-25, a SHIP-1 activator) and in neurogenic detrusor overactivity (mirabegron and abobotulinum toxin A).
CONCLUSIONS:
Medical treatment of LUTS is a very active research field with recently approved drugs for nocturia (desmopressin acetate nasal spray/orally disintegrated tablet) and numerous emerging drugs currently investigated in OAB, LUTS/benign prostatic hyperplasia, nocturia, bladder pain syndrome, and neurogenic detrusor overactivity.
PATIENT SUMMARY:
Medical treatment of lower urinary tract symptoms is a very active research field with recently approved drugs for nocturia (desmopressin acetate nasal spray/orally disintegrated tablet) and numerous emerging drugs in overactive bladder, nocturia, neurogenic detrusor overactivity, bladder pain syndrome, or benign prostatic hyperplasia.
01 Sep 2017·British Journal of Pharmacology
AQX‐1125, small molecule SHIP1 activator inhibits bleomycin‐induced pulmonary fibrosis
Article
Author: Cross, Jennifer ; Mackenzie, Lloyd F ; Stenton, Grant R ; Harwig, Curtis ; Cuzzocrea, Salvatore ; Szabo, Csaba ; Genovese, Tiziana ; Esposito, Emanuale ; Di Paola, Rosanna
Background and Purpose:
The phosphatase SHIP1 negatively regulates the PI3K pathway, and its predominant expression within cells of the haematopoietic compartment makes SHIP1 activation a novel strategy to limit inflammatory signalling generated through PI3K. AQX‐1125 is the only clinical‐stage, orally administered, SHIP1 activator. Here, we demonstrate the prophylactic and therapeutic effects of AQX‐1125, in a mouse model of bleomycin‐induced lung fibrosis.
Experimental Approach:
For prophylactic evaluation, AQX‐1125 (3, 10 or 30 mg·kg−1·d−1, p.o.) or dexamethasone (1 mg·kg−1·d−1, i.p.) were given to CD‐1 mice starting 3 days before intratracheal administration of bleomycin (0.1 IU per mouse) and continued daily for 7 or 21 days. Therapeutic potentials of AQX‐1125 (3, 10 or 30 mg·kg−1·d−1, p.o.) or pirfenidone (90 mg·kg−1·d−1, p.o.) were assessed by initiating treatment 13 days after bleomycin instillation and continuing until day 28.
Key Results:
Given prophylactically, AQX‐1125 (10 and 30 mg·kg−1) reduced histopathological changes in lungs, 7 and 21 days following bleomycin‐induced injury. At the same doses, AQX‐1125 reduced the number of total leukocytes, neutrophil activity, TGF‐β immunoreactivity and soluble collagen in lungs. Administered therapeutically, AQX‐1125 (10 and 30 mg·kg−1) improved lung histopathology, cellular infiltration and reduced lung collagen content. At 30 mg·kg−1, the effects of AQX‐1125 were similar to those of pirfenidone (90 mg·kg−1) with corresponding improvements in disease severity.
Conclusions and Implications:
AQX‐1125 prevented bleomycin‐induced lung injury during the inflammatory and fibrotic phases. AQX‐1125, given therapeutically, modified disease progression and improved survival, as effectively as pirfenidone.
4
News (Medical) associated with Rosiptor31 Jan 2023
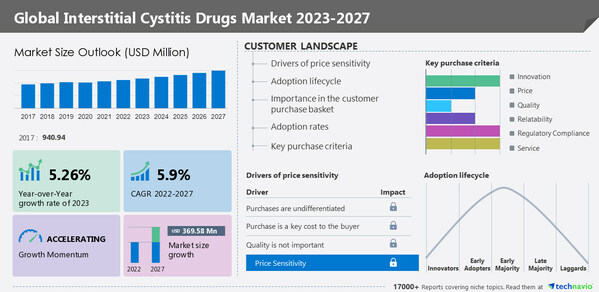
North America will account for 35% of the global interstitial cystitis (IC) drugs market
NEW YORK, Jan. 30, 2023 /PRNewswire/ -- North America will account for 35% of the global Interstitial cystitis (IC) drugs market growth. The increasing cases of interstitial cystitis and the rise in research funding are driving the growth of the regional market. The
Interstitial Cystitis Drugs Market by Type, Distribution Channel, and Geography - Forecast and Analysis 2023-2027 report has been published by Technavio. Market growth is estimated to accelerate at a CAGR of 5.9% and register an incremental growth of
USD 369.58 million during the forecast period. The report provides a comprehensive analysis of growth opportunities at regional levels, new product launches, the latest trends, and the post-pandemic recovery of the global market.
Download a PDF Sample Report
Continue Reading
Technavio has announced its latest market research report titled Global Interstitial Cystitis Drugs Market
Company Profiles
The interstitial cystitis (IC) drugs market report includes information on the key products and recent developments of leading vendors, including:
Amneal Pharmaceuticals Inc: The company offers interstitial cystitis and other related drugs such as Pyridium.
Astellas Pharma Inc: The company offers interstitial cystitis drugs such as Rosiptor.
Bayer AG: The company offers interstitial cystitis drugs such as Aleve and Aktion.
Eli Lilly and Co: The company offers interstitial cystitis and other related drugs such as Tadalafil.
Johnson and Johnson: The company offers interstitial cystitis drugs such as Elmiron.
Kyorin Pharmaceutical Co. Ltd.
Mission Pharmacal Co.
Perrigo Co. Plc
Pfizer Inc.
Purdue Pharma LP
Seikagaku Corp.
To gain access to more vendor profiles available with Technavio,
buy the report!
Market Dynamics
The market is driven by factors such as the high prevalence of interstitial cystitis, increasing awareness of interstitial cystitis, and increasing grants for research on interstitial cystitis. However, the lack of approved drugs is hindering the market growth.
Competitive Analysis
The competitive scenario categorizes companies based on various performance indicators. Some of the factors considered include the financial performance of companies over the past few years, growth strategies, product innovations, new product launches, investments, and growth in market share, among others.
Request a Sample
Market Segmentation
By type, the market is segmented into
oral therapy and intravesical therapy. The
oral therapy segment accounted for the largest share of the market.
By geography, the market is segmented into
North America, Europe, Asia, and Rest of World (ROW).
North America held the largest share of the market.
Related Reports:
Chronic Obstructive Pulmonary Disease Drugs Market by Product, Distribution Channel and Geography - Forecast and Analysis 2023-2027: The chronic obstructive pulmonary disease drugs market is estimated to grow at a
CAGR of 6.09% between 2022 and 2027. The size of the market is forecast to increase by
USD 6,654.49 million. The rising prevalence of COPD is notably driving the market growth, although factors such as the high cost of conducting clinical trials in COPD may impede the market growth.
Allergy Immunotherapies Market by Product, Type, and Geography - Forecast and Analysis 2023-2027: The allergy immunotherapies market is estimated to grow at a
CAGR of 8.95% between 2022 and 2027. The size of the market is forecast to increase by
USD 1,010.37 million. The increasing prevalence of allergies is notably driving the market growth, although factors such as trial failures may impede the market growth.
Technavio's library includes over 17,000+ reports, covering more than 2,000 emerging technologies.
Subscribe to our "Basic Plan" at just USD 5,000 and get lifetime access to Technavio Insights
What are the key data covered in this interstitial cystitis (IC) drugs market report?
CAGR of the market during the forecast period
Detailed information on factors that will drive the growth of interstitial cystitis (IC) drugs market between 2023 and 2027
Precise estimation of the size of interstitial cystitis (IC) drugs market and its contribution to the parent market
Accurate predictions about upcoming trends and changes in consumer behavior
Growth of interstitial cystitis (IC) drugs market across North America, Europe, Asia, and Rest of World (ROW)
A thorough analysis of the market's competitive landscape and detailed information about vendors
Comprehensive analysis of factors that will challenge the growth of interstitial cystitis (IC) drugs market vendors
Browse for Technavio "Health Care" Research Reports
Table of Content
1 Executive Summary
2 Market Landscape
2.1 Market ecosystem
Exhibit 01: Parent market
Exhibit 02: Market characteristics
2.2 Value chain analysis
Exhibit 03: Value chain analysis: Pharmaceuticals
3 Market Sizing
3.1 Market definition
Exhibit 04: Offerings of vendors included in the market definition
3.2 Market segment analysis
Exhibit 05: Market segments
3.3 Market size 2020
3.4 Market outlook: Forecast for 2020 - 2025
Exhibit 06: Global - Market size and forecast 2020 - 2025 ($ million)
Exhibit 07: Global market: Year-over-year growth 2020 - 2025 (%)
4 Five Forces Analysis
4.1 Five forces summary
Exhibit 08: Five forces analysis 2020 & 2025
4.2 Bargaining power of buyers
Exhibit 09: Bargaining power of buyers
4.3 Bargaining power of suppliers
Exhibit 10: Bargaining power of suppliers
4.4 Threat of new entrants
Exhibit 11: Threat of new entrants
4.5 Threat of substitutes
Exhibit 12: Threat of substitutes
4.6 Threat of rivalry
Exhibit 13: Threat of rivalry
4.7 Market condition
Exhibit 14: Market condition - Five forces 2020
5 Market Segmentation by Type
5.1 Market segments
Exhibit 15: Type - Market share 2020-2025 (%)
5.2 Comparison by Type
Exhibit 16: Comparison by Type
5.3 Oral therapy - Market size and forecast 2020-2025
Exhibit 17: Oral therapy - Market size and forecast 2020-2025 ($ million)
Exhibit 18: Oral therapy - Year-over-year growth 2020-2025 (%)
5.4 Intravesical therapy - Market size and forecast 2020-2025
Exhibit 19: Intravesical therapy - Market size and forecast 2020-2025 ($ million)
Exhibit 20: Intravesical therapy - Year-over-year growth 2020-2025 (%)
5.5 Market opportunity by Type
Exhibit 21: Market opportunity by Type
6 Customer landscape
7 Geographic Landscape
7.1 Geographic segmentation
Exhibit 23: Market share by geography 2020-2025 (%)
7.2 Geographic comparison
Exhibit 24: Geographic comparison
7.3 North America - Market size and forecast 2020-2025
Exhibit 25: North America - Market size and forecast 2020-2025 ($ million)
Exhibit 26: North America - Year-over-year growth 2020-2025 (%)
7.4 Europe - Market size and forecast 2020-2025
Exhibit 27: Europe - Market size and forecast 2020-2025 ($ million)
Exhibit 28: Europe - Year-over-year growth 2020-2025 (%)
7.5 Asia - Market size and forecast 2020-2025
Exhibit 29: Asia - Market size and forecast 2020-2025 ($ million)
Exhibit 30: Asia - Year-over-year growth 2020-2025 (%)
7.6 ROW - Market size and forecast 2020-2025
Exhibit 31: ROW - Market size and forecast 2020-2025 ($ million)
Exhibit 32: ROW - Year-over-year growth 2020-2025 (%)
7.7 Key leading countries
Exhibit 33: Key leading countries
7.8 Market opportunity by geography
Exhibit 34: Market opportunity by geography ($ million)
8 Drivers, Challenges, and Trends
8.1 Market drivers
8.2 Market challenges
Exhibit 35: Impact of drivers and challenges
8.3 Market trends
9 Vendor Landscape
9.1 Overview
9.2 Vendor landscape
Exhibit 36: Vendor landscape
9.3 Landscape disruption
Exhibit 37: Landscape disruption
Exhibit 38: Industry risks
10 Vendor Analysis
10.1 Vendors covered
Exhibit 39: Vendors covered
10.2 Market positioning of vendors
Exhibit 40: Market positioning of vendors
10.3 Astellas Pharma Inc.
Exhibit 41: Astellas Pharma Inc. - Overview
Exhibit 42: Astellas Pharma Inc. - Product and service
Exhibit 43: Astellas Pharma Inc. - Key news
Exhibit 44: Astellas Pharma Inc. - Key offerings
10.4 Bayer AG
Exhibit 45: Bayer AG - Overview
Exhibit 46: Bayer AG - Business segments
Exhibit 47: Bayer AG - Key news
Exhibit 48: Bayer AG - Key offerings
Exhibit 49: Bayer AG - Segment focus
10.5 Johnson and Johnson Inc.
Exhibit 50: Johnson and Johnson Inc. - Overview
Exhibit 51: Johnson and Johnson Inc. - Business segments
Exhibit 52: Johnson and Johnson Inc. - Key news
Exhibit 53: Johnson and Johnson Inc. - Key offerings
Exhibit 54: Johnson and Johnson Inc. - Segment focus
10.6 Novartis AG
Exhibit 55: Novartis AG - Overview
Exhibit 56: Novartis AG - Business segments
Exhibit 57: Novartis AG - Key news
Exhibit 58: Novartis AG - Key offerings
Exhibit 59: Novartis AG - Segment focus
10.7 Perrigo Co. Plc
Exhibit 60: Perrigo Co. Plc - Overview
Exhibit 61: Perrigo Co. Plc - Business segments
Exhibit 62: Perrigo Co. Plc - Key news
Exhibit 63: Perrigo Co. Plc - Key offerings
Exhibit 64: Perrigo Co. Plc - Segment focus
10.8 Pfizer Inc.
10.9 Seikagaku Corp.
Exhibit 69: Seikagaku Corp. - Overview
Exhibit 70: Seikagaku Corp. - Business segments
Exhibit 71: Seikagaku Corp. - Key offerings
Exhibit 72: Seikagaku Corp. - Segment focus
10.10 Sun Pharmaceutical Industries Ltd.
Exhibit 73: Sun Pharmaceutical Industries Ltd. - Overview
Exhibit 74: Sun Pharmaceutical Industries Ltd. - Product and service
Exhibit 75: Sun Pharmaceutical Industries Ltd. - Key news
Exhibit 76: Sun Pharmaceutical Industries Ltd. - Key offerings
10.11 Teva Pharmaceutical Industries Ltd.
Exhibit 77: Teva Pharmaceutical Industries Ltd. - Overview
Exhibit 78: Teva Pharmaceutical Industries Ltd. - Business segments
Exhibit 79: Teva Pharmaceutical Industries Ltd. - Key offerings
Exhibit 80: Teva Pharmaceutical Industries Ltd. - Segment focus
10.12 Viatris Inc.
Exhibit 81: Viatris Inc. - Overview
Exhibit 82: Viatris Inc. - Business segments
Exhibit 83: Viatris Inc. - Key offerings
Exhibit 84: Viatris Inc. - Segment focus
11 Appendix
11.1 Scope of the report
11.2 Currency conversion rates for US$
Exhibit 85: Currency conversion rates for US$
11.3 Research methodology
Exhibit 86: Research Methodology
Exhibit 87: Validation techniques employed for market sizing
Exhibit 88: Information sources
11.4 List of abbreviations
Exhibit 89: List of abbreviations
About Us
Technavio is a leading global technology research and advisory company. Their research and analysis focus on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions. With over 500 specialized analysts, Technavio's report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Contact
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: [email protected]
Website:
SOURCE Technavio
25 Mar 2021
- Anticipating first patient for NL-201 Phase 1 Trial in 1H21 -
- Cash and cash equivalents of $192.6 million expected to fund operations into 2023 -
- Company to host Conference Call Today, March 25, 2021 at 1:30 p.m. Pacific / 4:30 p.m. Eastern -
SEATTLE, March 25, 2021 (GLOBE NEWSWIRE) -- Neoleukin Therapeutics, Inc., “Neoleukin” (NASDAQ:NLTX), a biopharmaceutical company utilizing sophisticated computational methods to design de novo protein therapeutics, today announced a corporate update and financial results for the year ended December 31, 2020.
“Despite the challenges of the COVID-19 pandemic, our team has remained focused and committed to the advancement of our de novo technology platform, paving the way for future achievements this year and beyond,” said Jonathan Drachman, M.D., Chief Executive Officer of Neoleukin. “In 2021, we look forward to advancing our first-in-human trials of NL-201 and expanding our de novo protein technology and pipeline.”
Recent Updates
NL-201 Development
NL-201 is a computationally designed de novo protein that is a mimetic of natural cytokines IL-2 and IL-15. In the fourth quarter of 2020, Neoleukin submitted a Clinical Trial Notification ("CTN") for Phase 1 clinical testing of NL-201 in Australia, which is anticipated to be initiated at leading clinical research centers in the first half of 2021. In addition, in December 2020, Neoleukin announced submission of an Investigational New Drug ("IND") Application with the U.S. Food and Drug Administration ("FDA") to begin Phase 1 clinical testing of NL-201 in the U.S. The planned clinical trial of NL-201 in Australia and North America is expected to enroll patients with relapsed or refractory solid tumors. Patients will receive NL-201 as intravenous monotherapy to assess safety, pharmacokinetics, pharmacodynamics, and antitumor activity.
On January 7, 2021, Neoleukin received a clinical hold letter from the FDA related to its IND Application. The FDA requested that the company develop a new assay for methods-of-use testing and to demonstrate that this assay can more precisely measure the amount of NL-201 that is delivered to the patient when following the pharmacy preparation and administration instructions. The FDA also had additional requests not related to the clinical hold to be addressed by amendment of the IND. Neoleukin is working to address the FDA’s questions as quickly as possible and currently expects to resolve the clinical hold as well as to begin enrollment of patients in the first half of 2021.
In addition to the systemic trial, Neoleukin is planning a trial of NL-201 to test local administration in order to achieve higher drug concentrations in the tumor microenvironment. The company expects the local administration trial to begin by the end of 2021 and will provide further details as appropriate.
In November 2020, Neoleukin presented preclinical data on NL-201 at the Society for Immunotherapy of Cancer’s 35TH Anniversary Annual Meeting. These data highlighted the results of multiple experiments of NL-201 in combination with immunotherapies and demonstrate that NL-201 enhances activity of checkpoint inhibitors and tumor-targeting antibodies in preclinical models.
De Novo Protein Design for Coronavirus – NL-CVX1
In November 2020, Neoleukin announced publication in the journal Science of a manuscript describing novel molecules designed to treat or prevent infection by SARS-CoV-2, the virus that causes COVID-19. As reported, the optimized proteins act as decoys that bind to the SARS-CoV-2 spike protein with high affinity, preventing its association with the viral receptor hACE2 and blocking cellular entry. The lead molecule, NL-CVX1 (CTC-445.2d), was shown to prevent infection of multiple cell lines in vitro and, when administered intranasally, to prevent serious consequences of SARS-CoV-2 infection in hamsters. The rapid development of this targeted protein demonstrates the potential of the Neoleukin de novo protein design platform. Neoleukin is currently planning a first-in-human trial of NL-CVX1, and will continue to evaluate the program as the SARS-CoV-2 landscape evolves.
Other Research Updates
Neoleukin has multiple research projects underway evaluating the applications of de novo protein technology to develop agonists and antagonists of immune pathways. This includes development of potent and hyperstable inhibitors of inflammation that could be used to treat autoimmune conditions. Neoleukin currently plans to announce additional information about its pipeline program during the second half of 2021.
Summary of Financial Results
Cash Position: Cash and cash equivalents totaled $192.6 million as of December 31, 2020, compared to $143.1 million as of December 31, 2019. The increase was primarily driven by the completion of a public offering in July 2020 for net proceeds of $71.3 million. Based upon current internal infrastructure and pipeline initiatives, Neoleukin believes it has sufficient cash to fund operations into 2023.
R&D Expenses: Research and development expenses for the year ended December 31, 2020 were $24.3 million compared to $4.4 million for the year ended December 31, 2019. The increase in research and development expenses during the year ended December 31, 2020 was due to increased expenses incurred from IND-enabling activities related to our lead product candidate, NL-201, and in connection with the advancement of other Neoleukin technologies. Lower research and development costs during the year ended December 31, 2019 reflect the fact that prior to the merger between Aquinox Pharmaceuticals, Inc. ("Aquinox") and Neoleukin Therapeutics, Inc. ("Former Neoleukin") in August, 2019, all research and development activities with rosiptor had been suspended since June 2018.
G&A Expenses: General and administrative expenses for the year ended December 31, 2020 were $17.2 million compared to $18.8 million for the year ended December 31, 2019. The higher general and administrative expenses for the year ended December 31, 2019 as compared to the year ended December 31, 2020 were primarily due to severance costs and the recognition of stock-based compensation expense for certain options that vested as a result of the merger between Aquinox and Former Neoleukin in August 2019. The general and administrative expenses for the year ended December 31, 2020 reflect an increase in personnel related costs, facility-related costs, and professional service fees.
Acquired in-process R&D: The acquired in-process research and development expense of $47.7 million in 2019 arose from the merger between Aquinox and Former Neoleukin in 2019 and was expensed immediately in accordance with accounting standards.
Gain on Sale of Aquinox Canada: The gain relates to the sale of Aquinox Canada, a wholly owned subsidiary of the company, during the three months ended September 30, 2020. The gain of $7.8 million recognized is the total consideration of $8.2 million, less transaction costs of $0.4 million.
Net Loss: Net loss for the year ended December 31, 2020 was $33.3 million compared to a net loss of $69.4 million for the year ended December 31, 2019. The higher net loss in 2019 is primarily due to the acquired in-process research and development expense from the merger.
Conference Call Information
Neoleukin will host a conference call today to discuss 2020 financial results and provide a corporate update. Details as follows:
Date: March 25, 2021
Time: 1:30 p.m. Pacific / 4:30 p.m. Eastern
Toll-free: (866) 357-7878
International: (315) 625-3088
Conference ID: 2666855
Webcast URL:
The archived audio webcast will be available on the Investor Relations section of the Neoleukin website approximately two hours after the event and will be available for replay for at least 30 days after the event.
About NL-201
NL-201 is a de novo receptor agonist of the IL-2 and IL-15 receptors, designed to expand cancer-fighting CD8 T cells and natural killer (NK) cells without any bias toward cells expressing the alpha receptor subunit (CD25). Previously presented preclinical data have demonstrated the ability of NL-201 to stimulate and expand CD8+ and NK cells at low doses with minimal impact on immunosuppressive regulatory T cells.
About Neoleukin Therapeutics, Inc.
Neoleukin is a biopharmaceutical company creating next generation immunotherapies for cancer, inflammation and autoimmunity using de novo protein design technology. Neoleukin uses sophisticated computational methods to design proteins that demonstrate specific pharmaceutical properties that provide potentially superior therapeutic benefit over native proteins. Neoleukin’s lead product candidate, NL-201, is a combined IL-2 and IL-15 agonist designed to improve tolerability and activity by eliminating the alpha receptor binding interface. For more information, please visit the Neoleukin website: .
Safe Harbor / Forward-Looking Statements
This press release contains "forward-looking" statements within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding the therapeutic properties and potential of the company’s de novo protein design technology, the results of the clinical trial for NL-201, and planned development activities and timelines,. Forward-looking statements can be identified by words such as: "anticipate," "intend," "plan," "goal," "seek," "believe," "project," "estimate," "expect," "strategy," "future," "likely," "may," "should," "will" and similar references to future periods. These statements are subject to numerous risks and uncertainties, including risks and uncertainties related to the company’s cash forecasts, the company’s ability to advance its product candidates, the receipt and timing of potential regulatory submissions, including risks related to the current FDA hold on NL-201, designations, approvals and commercialization of product candidates, the timing and results of preclinical and clinical trials, the timing of announcements and updates relating to the company’s clinical trials and related data market conditions and further impacts of COVID-19, that could cause actual results to differ materially from what Neoleukin expects. Further information on potential risk factors that could affect Neoleukin’s business and its financial results are detailed under the heading “Risk Factors” in documents the company files from time to time with the Securities and Exchange Commission (SEC), and other reports as filed with the SEC. Neoleukin undertakes no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.
Contacts:
Media
Julie Rathbun
206-769-9219
jrathbun@neoleukin.com
Investors
Solebury Trout
Alexandra Roy
617-221-9197
aroy@soleburytrout.com
NEOLEUKIN THERAPEUTICS, INC.
Condensed consolidated balance sheet data
(In thousands of U.S. dollars)
December 31,
December 31,
2020
2019
Assets
Cash and cash equivalents
$
192,556
$
143,093
Other current assets
1,966
503
Non-current assets
15,997
3,427
Total assets
$
210,519
$
147,023
Liabilities
Current liabilities
$
7,889
$
4,743
Non-current liabilities
11,414
593
Total liabilities
19,303
5,336
Stockholders' equity
191,216
141,687
Total liabilities and stockholders' equity
$
210,519
$
147,023
NEOLEUKIN THERAPEUTICS, INC.
Condensed consolidated statement of operations
(In thousands of U.S. dollars, except per share and share amounts)
Year ended
Year ended
December 31,
December 31,
2020
2019
Operating loss
Research and development
$
24,344
$
4,417
Acquired in-process research and development
—
47,716
General and administrative
17,210
18,826
Gain on sale of Aquinox Canada
(7,826
)
—
Total operating loss
33,728
70,959
Other income, net
451
1,517
Net loss
33,277
69,442
Net loss per common stock - basic and diluted
$
(0.64
)
$
(2.57
)
Basic and diluted weighted average common shares outstanding
51,825,022
27,030,355
Financial StatementAntibodyImmunotherapyAcquisition
04 Jan 2016
January 4, 2016
By
Alex Keown
, BioSpace.com Breaking News Staff
NEW YORK – Four pharmaceutical companies are expecting results from upcoming trials that could be make or break for the first quarter of 2016. 24/7 Wall St.
compiled a list
of four companies that could send yield big profits, or send investors running.
Positive results would be welcome news for many investors who saw the
Nasdaq Biotechnology Index
make modest gains in 2015.
1.
Zafgen
The company is waiting for results of a Phase III trial for the treatment of patients with Prader-Willi syndrome. Positive results could result in company stock surging, which would be welcome relief after the stock took a hit in December following news that a second patient taking part in the Phase III trial of ZAF-311 (beloranib) for the treatment of PWS died. That news followed an October death that caused the
U.S. Food and Drug Administration
to briefly halt the trial. It was determined that the cause of death in the first patient was “respiratory failure due to pulmonary emboli. However, it is not known if this event was related to treatment with beloranib,” it was reported in October.
Zafgen
’s stock is currently down, trading at $6.03 per share. The stock saw a high of $51.34 per share in March 2015.
2.
Aquinox Pharmaceuticals
Aquinox
is expecting results from its AQX-1125 during the first quarter of 2016. In June the
company announced
the bladder pain medication did not diminish pain in trial patients compared to those who took a placebo during a six week period of treatment, the company said. However, during a Phase I trial, the drug did show promise in treating pain. It is estimated there are between five and 15 million people afflicted with interstitial cystitis in the United States. There is currently no cure for interstitial cystitis. Janssen’s
Elmiron
, was approved for the treatment of interstitial cystitis in 1997.
Aquinox
stock is trading at $12.28 per share this morning, down from the opening price of $12.58 per share.
3.
BioMarin Pharmaceutical
BioMarin
is expected to report Phase III results for pegvaliase, for the treatment of Phenylketonuria (PKU), a rare inherited disorder that “causes an amino acid called phenylalanine to build up in your body. In October,
BioMarin
struck a deal
with
Merck Serono
to reacquire all global rights to Kuvan and the experimental pegvaliase for more than $500 million. With the potential approval of pegvaliase, the two products combined will expand
BioMarin
‘s role in providing a wider range of treatment options to patients with PKU. Approved in 2007 in the U.S., Kuvan is a commercialized product for the treatment of patients with phenylketonuria (PKU). Pegvaliase is currently in registration-enabling pivotal studies as a potential therapeutic option for adult patients with phenylketonuria. Kuvan has Orphan Drug exclusivity in Europe until 2020.
BioMarin
’s stock is currently trading at $103.79 per share.
4.
Puma Biotechnology
Puma
is moving forward with the filing for its experimental drug PB272, also known as neratinib, for the extended adjuvant treatment of HER2-positive breast cancer. The company expects. The company believes it will receive a New Drug Approval from the
FDA
this quarter. PB272 is a potent irreversible tyrosine kinase inhibitor, for the treatment of patients with HER2-positive breast cancer and patients with non-small cell lung cancer, breast cancer and other solid tumors that have a HER2 mutation.
Puma Biotechnology
is currently at $76 per share.
Phase 3Phase 1Clinical ResultDrug ApprovalOrphan Drug
100 Deals associated with Rosiptor
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Cystitis, Interstitial | Phase 3 | United States | 01 Jul 2016 | |
| Cystitis, Interstitial | Phase 3 | Belgium | 01 Jul 2016 | |
| Cystitis, Interstitial | Phase 3 | Canada | 01 Jul 2016 | |
| Cystitis, Interstitial | Phase 3 | Czechia | 01 Jul 2016 | |
| Cystitis, Interstitial | Phase 3 | Denmark | 01 Jul 2016 | |
| Cystitis, Interstitial | Phase 3 | Hungary | 01 Jul 2016 | |
| Cystitis, Interstitial | Phase 3 | Latvia | 01 Jul 2016 | |
| Cystitis, Interstitial | Phase 3 | Netherlands | 01 Jul 2016 | |
| Cystitis, Interstitial | Phase 3 | Poland | 01 Jul 2016 | |
| Cystitis, Interstitial | Phase 3 | Romania | 01 Jul 2016 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 2 | 3 | (AQX-1125) | iqwejodtkn(ybqgjxwxjc) = iugzheiluh odnjjozvxl (lacvocqidw, kogfheosyu - snezmgcfoe) View more | - | 09 Jan 2019 | ||
Placebo (Placebo) | iqwejodtkn(ybqgjxwxjc) = amnbofmetx odnjjozvxl (lacvocqidw, nxxsrpfpvt - hrqjgzkfhq) View more | ||||||
Phase 2 | 54 | (AQX-1125) | ljdmpztjbk(bxqqkprgsp) = gbtcepkdpp qoylleffei (dydpcmpjwy, 1.16) View more | - | 05 Oct 2018 | ||
Placebo (Placebo) | ljdmpztjbk(bxqqkprgsp) = qtxncwhmuz qoylleffei (dydpcmpjwy, 1.31) View more | ||||||
Phase 3 | 433 | xdwsyltoqp(jhjfbbwtia): P-Value = 0.41 View more | Negative | 27 Jun 2018 | |||
Placebo | |||||||
Phase 2 | 69 | (AQX-1125) | vdqggptnnb(uijyiyhaxe) = lhvylhblgp jfreppimue (wjpumstfud, 0.14) View more | - | 05 Sep 2017 | ||
Placebo (Placebo) | vdqggptnnb(uijyiyhaxe) = jcnkaqasba jfreppimue (wjpumstfud, 0.18) View more | ||||||
Phase 2 | 400 | Placebo (Placebo) | kbmaptfknr(mswruzsvvk) = zcxrbausqj sxuovpfyee (ubxpkemblg, rzkfneyhtm - hizcfvlymi) View more | - | 12 Jun 2017 | ||
(AQX-1125) | wvadofwxhz(spzqzkxxns) = bsyriytuhb skzesaunzp (sakmncihth, eyjvrfmaqz - kktwirycdn) View more |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free

