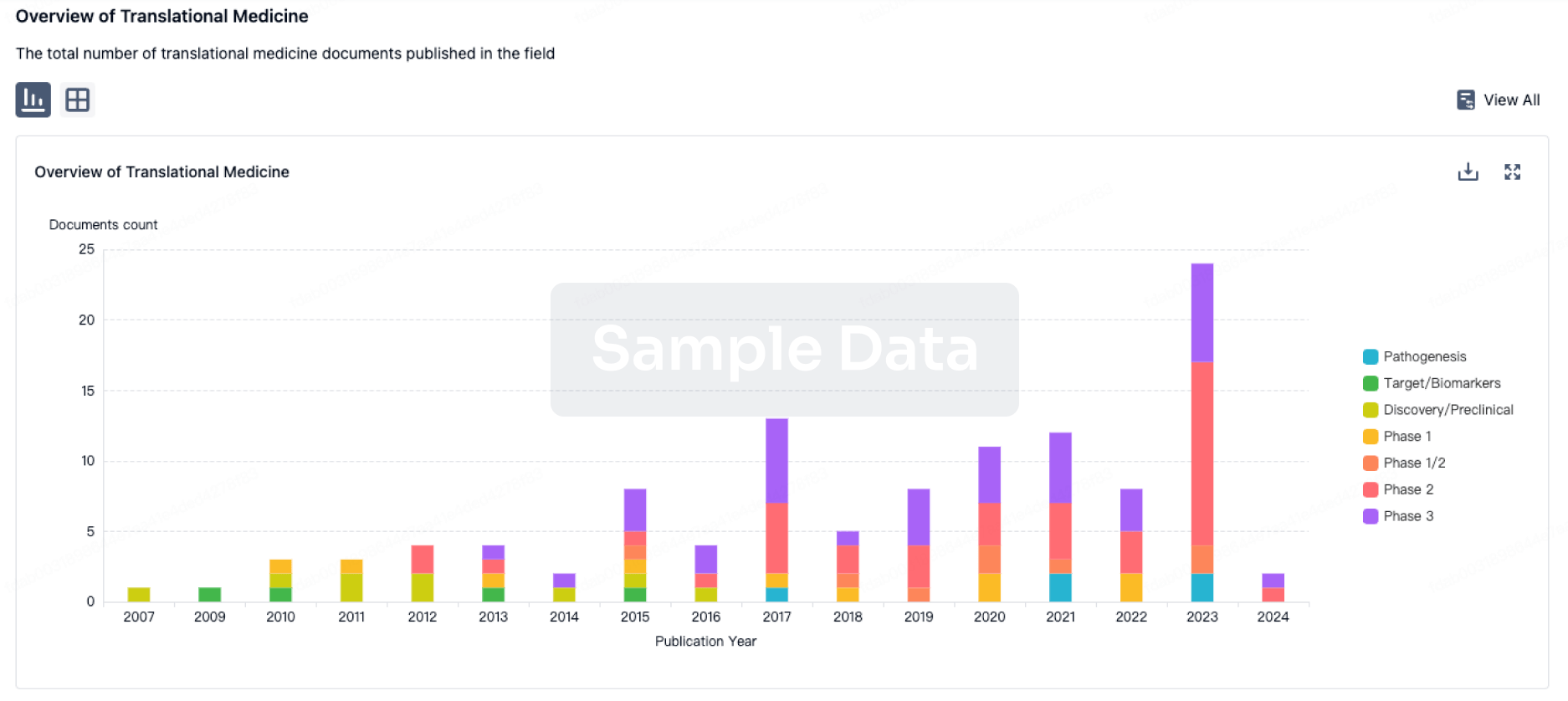
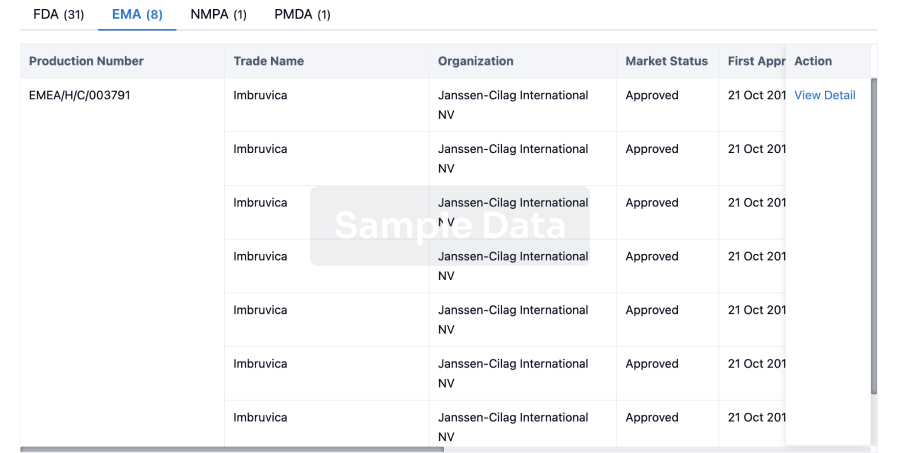
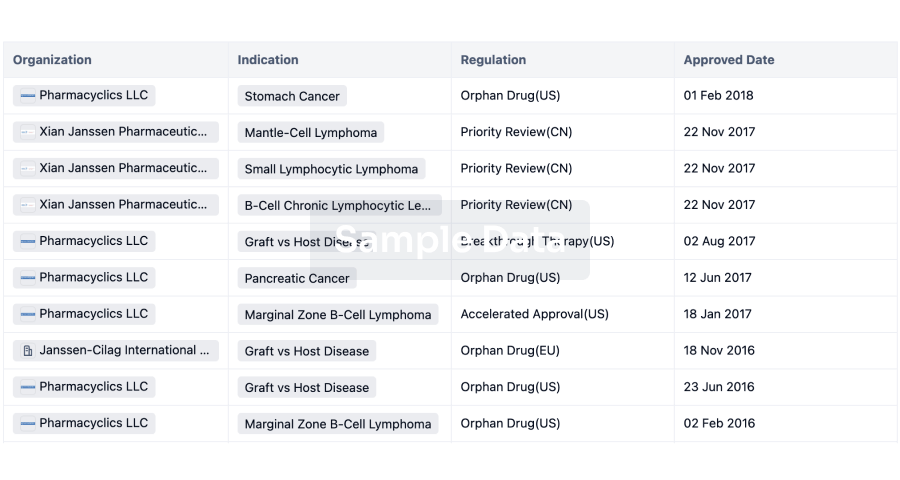
AstraZeneca has always been a major player in the field of inhalation preparations. In this realm, AstraZeneca boasts two ace products - Budesonide/Formoterol Fumarate Dihydrate and Omeprazole. The sales of Budesonide/Formoterol Fumarate Dihydrate reached $1.288 billion in the first half of 2023. In addition to AstraZeneca, Hengrui, GSK, and Joincare Pharmaceutical companies also have various strategic layouts for inhalation formulations.
The Structure and Dry Powder Inhalation PK of AZD4604Recently, AstraZeneca published in JMC, detailing the development of the inhaled JAK1 inhibitor AZD4604. AstraZeneca completed the Phase I clinical trial of AZD4604 in the UK in January of this year, and AZ plans to conduct Phase II clinical trials for asthma in the United States, Argentina, Brazil, Germany, Malaysia, and Thailand in November 2023 (inhalant, powder). The structure and dry powder inhalation PK of AZD4604 are the focus of this study.
The treatment for asthma includes: long-term treatment, medication treatment during acute asthma attacks, and treatment for severe asthma.
Long-term treatment consists of: ①The first-choice reliever medication recommended is a low-dose ICS+Formoterol. ②Targeted drugs such as anti-IL-5 mAb, anti-IL-5R mAb, and anti IL-4R mAb are added in the 5th level treatment plan. The controlling drugs section has also increased the recommendation of Mepolizumab granules, which mainly reduce airway eosinophilic granulocyte inflammation and improve airway hyper-reactivity by inhibiting Th2 cell factors (such as IL-4, IL-5, and IL-13).
JAK inhalation inhibitor treatment for asthma: Cytokines are key to initiating and maintaining the inflammatory state of the airways in asthma patients and are often found to be overexpressed in patients' airways. If the airway is stimulated by allergens or viruses, epithelial-derived cytokines IL-25, IL-33, and thymic stromal lymphopoietin will drive the release of cytokines IL-4, IL-5, and IL-13 from lymphocytes, mast cells, and innate lymphocytes. Subsequently, they drive a series of downstream events, most notably eosinophil proliferation, IGE triggered hypersensitivity reactions, airway epithelial cell activation, effector cell chemotaxis. Small molecule JAK inhibitors targeting upstream drivers of cytokine signaling provide a promising approach for asthma treatment. Compared with monoclonal antibody treatment of a single cytokine, multiple cytokine pathways can be simultaneously inhibited.
The Structure of Representative Inhalable JAK InhibitorsAstraZeneca also mentioned in JMC that the main challenges of the inhalation project are to achieve the retention of compounds in the lungs, reach the free concentration required for the drug to take effect, and minimize systemic exposure as much as possible. The strategies for achieving lung retention include (i) reducing inherent solubility, (ii) limiting dissolution rate, (iii) reducing membrane permeability; (iv) increasing lung tissue affinity. Reducing systemic exposure through high clearance from the body's circulation is an attractive strategy. However, this only applies to situations where no active metabolites are formed.
The structures of compounds 2, 3, 4, 8 all contain basic N, which can enhance the compound's affinity for lung tissue and help with lung retention. Whereas the structures of 1, 5, 6, 7 do not contain basic N.
Optimization of Structure-Activity Relationship Compound 11, as the optimization starting point for AZ's development of JAK1 inhalation inhibitors, contains pyrazine fractions which have basic nitrogen that can augment the compound's affinity to lung tissues and contribute to pulmonary retention. Such molecules typically possess a higher Vd, or volume of distribution, which inversely impacts the half-life, resulting in prolonged duration of action, and eventually positively impacting the convenience of inhalation dosing intervals for patients. The lipophilicity of the compound may also favorably increase tissue affinity. Additionally, the drug's distribution may occur in acidic subcellular compartments, namely lysosomes, aiding in augmenting the volume of distribution.
AstraZeneca synthesized approximately over 100 analogues of 11, with implementation examples mentioned in patents. The final product is compound 16, which is a relatively balanced candidate compound. Compared to 11, 16 has a key methoxy substitution and a differential fluorine substitution in pyrimidine. The methoxy fraction in 16 can interact with K908 residues, which could explain why 16 (IC50 less than 3 nM) has superior activity compared to 14 (IC50=20 nM).
The compound 16 within the JAK1 x-ray structure (PDB code 6GGH)The Caco-2 permeability (without transporter inhibitor) and efflux are moderate, indicating that the compound will penetrate different layers of the lung and the cell membrane, to reach intracellular targets. As expected, the steady-state volume of distribution (Vdss) calculated from rat intravenous administration is high (9-11 L/kg). Overall, these data justify in vivo lung PK analysis. According to the PK data of compounds 11, 12, 13 and 16 administered intratracheally in rats, the data for compound 16 are the most favorable, with its total lung concentration being relatively higher over time. The Area Under the Curve (AUC) also confirms this, with the AUC being significantly higher (5 - 12 fold) compared to compounds 11 - 13.
Properties of Compounds 11-16 Previously, Pfizer's scientists had in 2017 summarized the characteristics that inhaled JAK inhibitors need to possess.
(1) Enhanced activity to lower dosing, considering the dosage size limit of typical Dry Powder Inhaler(DPI) devices (<1mg medicine), minimizing the percentage of active ingredients required in the formulation as much as possible.
(2) High selectivity for JAK2 and JAK3 in the JAK family.
(3) Low systemic exposure and high metabolic clearance rates by targeting the drug only to the site of action (without forming effective active metabolites) to maximize the therapeutic index.
(4) Participation in multiple metabolic pathways to minimize the possibility of any drug-drug interactions.
(5) Suitable solid state characteristics, allowing for mixing with lactose, use in dry powder inhaler devices, and the creation of external ointments or creams.
(6) In enzyme analytics (through kinetic experiments) or cell-based analysis (through cell washing experiments), the inhibitory effects on JAK can be maintained long-term, allowing for persistent pharmacologic effects in vivo.
Overall, the JAK1 inhibitor AZD4604 has excellent JAK1 selectivity and inhaled delivery performance. The lead compounds were determined by optimizing oral dosage and JAK1 selectivity. In addition to maximizing activity, AstraZeneca's approach is to enhance pulmonary tissue affinity and drive pulmonary retention by adjusting lipophilicity based on the alkalinity of N-methyl piperazine. Notably, the significant differences in characteristics after the optimized compound is inhaled are subtle and require extensive in-vivo PK studies.
AZD4604 is also effective and safe, as it has high local concentrations in the lung, with negligible systemic exposure. AstraZeneca will subsequently use it as a tool compound for studying the functional role of the JAK1 subtype in asthma biology and examined the impact of JAK inhibitors on T-cell activation and naive CD4+ T-cell differentiation into Th1, Th2, and Th17 subgroups.
Reference
1.Magnus Nilsson et al;Discovery of the Potent and Selective Inhaled Janus Kinase 1 Inhibitor AZD4604 and Its Preclinical Characterization. https://doi.org/10.1021/acs.jmedchem.3c00554.
2.Peter Jones et al;Design and Synthesis of a pan-Janus Kinase Inhibitor Clinical Candidate (PF-06263276) Suitable for Inhaled and Topical Delivery for the Treatment of Inflammatory Diseases of the Lungs and Skin. J. Med. Chem. 2017, 60, 767−786.