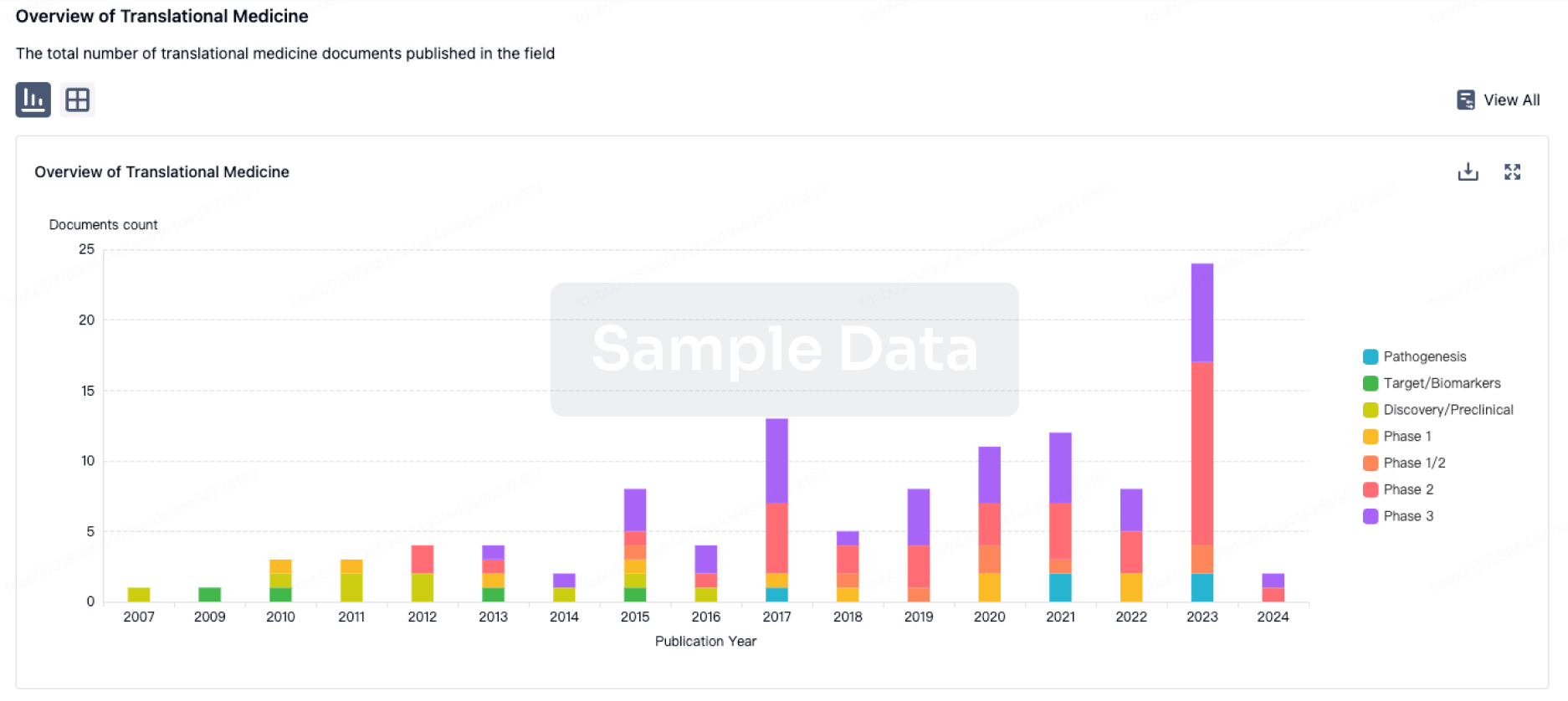
Claudin 6 T Cell engagers: A novel strategy for solid tumorsAntibody immunotherapy has revolutionized oncology treatment for melanoma, lung, stomach, cervical, and certain types of breast cancer. While effective for many patients, current immunotherapy approaches are broad-acting, turning off or on entire populations of immune cells. This approach to systemic immune modulation can lead to debilitating autoimmune or bystander side effects for many patients. An alternative to non-selective immunotherapy is precision targeting which is intended to only activate immune cells within the tumor microenvironment and reduce the risk of systemic side effects. T cells are a type of white blood cell that play a central role in coordinating immune responses. One of their functions is to recognize and kill tumors, however tumor growth happens in part because the T cells dont effectively recognize the tumors. T cell engaging bispecific antibodies (TCE) are potent tumor-targeting agents that act by linking a patients T cells to proteins expressed on a cancer cell surface to create an augmented recognition that allows the T cells to kill the cancer cells. TCEs have had tremendous clinical success targeting blood cancers that express protein-based antigens including CD19, CD20, or B-cell maturation antigen (BCMA). However, TCE success in solid tumors has been limited, with only one treatment (Kimmtrak, tebentafusp) approved by the U.S. Food and Drug Administration to date. However, as researchers learn more about TCE and how best to use them, there is reason for optimism.Lessons learned and a path forwardDrug development is an iterative process and past failures for TCEs in solid tumors help guide product refinement for todays emerging TCE products. Compared to treating blood cancers, solid tumors require much higher doses of TCE to ensure enough T cells can enter the tumor microenvironment. However, increasing the peripheral exposure of the TCE in solid tumors heightens the risk of off-target toxicities. TCEs in solid tumors have historically been challenged by systemic spillover of the induced immune responses, including cytokine release syndrome (CRS), an acute systemic inflammatory syndrome that in extreme cases can lead to multi-organ failure. As the understanding of CRS has evolved, several critical risk factors have been identified that can be mitigated with improved target selection, TCE engineering, and by tailoring the pharmacokinetic profile of the TCE. The recent approval of Kimmtrak in uveal melanoma and promising early clinical data in small cell lung cancer programs targeting the DLL3 protein (AMG757, BI764532, HPN328) reflects this more refined understanding of TCE and unlocks the potential for TCE in solid tumor therapy.CRS tttRisk FactorCase StudiesMitigation StrategyTarget ExpressionEpCAM (AMG110), CEA (cibisatamab), PSMA (HPN424, AMG160), HER2 (Ertumaxomab, GBR1302), and SSTR2 (XmAb18087) experiencedttton-target/off-tumor toxicityTargets selectively expressed in cancer cells (CLDN6, DLL3) and/or increase target avidityADCC and CDC ActivityEpCAMxCD3 (HO-3) wildtype Fc with full effector functions resulted in significant toxicity in Phase 1Silence Fc or remove Fc domainAntidrug antibody (ADA) CEA (AMG211) and PSMA (MOR209) bispecifics induced aggregate formation and/or T cell scaffolding Target selection; fully humanized antibodyFast onset of actionCD20 (mosunetuzumab) and glypican 3 (ERY974) induced spikes in TCE concentration (Cmax)Utilize extended half-life TCE, lower/more frequent dose, or step-up dosing schemeFuture of TCE developmentThe early clinical success of TCE programs targeting DLL3 provides hope that other promising tumor antigens could achieve similar clinical outcomes. A critical determinant of this programs success may be that DLL3 expression is highly restricted to cancer cells. This minimizes off-tumor activation of T cells and the systemic toxicities that have been seen in some other TCE programs. Because other kinds of tumors will display different patterns of tumor antigens, identifying other cancer targets with similarly tumor-restricted patterns of expression is of significant interest to the cancer research community.One such emerging target of interest is Claudin 6 (CLDN6). CLDN6 is an embryonic gene that is turned on during fetal development and then silenced after birth, thus not expressed or expressed at low levels in normal healthy cells. Embryonic genes are often epigenetically reactivated by cancer cells as a survival mechanism to support the rapid growth of the tumor. Unlike DLL3, CLDN6 is enriched in a wide range of cancers, including non-small cell lung, testicular, and ovarian. Overall, it is estimated that there are 62,500 patients with metastatic CLDN6-positive cancer in the United States.The success of CLDN6-targeted TCE approaches will depend on the ability to optimize TCE engineering and dosing schedule to further minimize the risk of CRS. Two contrasting approaches are exemplified by CTIM-76 (Context Therapeutics) and AMG794 (Amgen). The Context and Amgen TCEs have both adopted FcRn-binding strategies to extend half-life and improve PK.Key to their success will be their ability to specifically target tumor-associated CLDN6 and the efficiency with which they can recruit T cells and their effector functions.CTIM-76AMG794Antibody FormatFull lengthFragmentFcR BindingReducedNoCD3 FormatMonovalent anti-CD3 scFvMonovalent anti-CD3 scFvDosingPotentially every 2-3 weeksWeeklyDefining the pathway to success for TCE in solid tumors has recently been solidified with a clear focus on optimization of TCE antibody engineering and TCE pharmacokinetics, and a selection of tumor-restricted targets. With these risk determinants in hand, TCEs are poised to have a significant impact on the treatment of solid tumors in the coming years. '