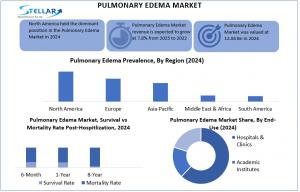
Pulmonary Edema revenue is expected to grow at a CAGR of 7.8 % from 2025 to 2032, reaching nearly USD 12.04 bn by 2032.
Surging heart failure, chronic lung disorders, and trauma cases are propelling the Pulmonary Edema Market, with advanced therapies and diagnostics driving global healthcare innovation.”
— Navneet Kaur
MIAMI, FL, UNITED STATES, October 16, 2025 /
EINPresswire.com
/ --
Explore the booming
Pulmonary Edema Market
, valued at USD 6.6 Bn in 2024 and set to reach USD 12.04 Bn by 2032. Discover key growth trends, rising cardiovascular disease cases, advanced diagnostics, oxygen therapy, gene therapies, and market insights driving global expansion and investment opportunities.
Pulmonary Edema Market Overview:
Pulmonary Edema Market, valued at USD 6.6 Bn in 2024 and projected to reach USD 12.04 Bn by 2032, is booming due to rising cardiovascular diseases, heart failure, chronic lung disorders, and trauma-related injuries. North America leads with advanced healthcare and key players like Pfizer, Novartis, ResMed, and AstraZeneca driving innovation through FDA approvals, gene therapies, and strategic acquisitions. Emerging markets such as China and India offer growth opportunities, while AI-assisted diagnostics, oxygen therapy, and novel treatments enhance patient outcomes, making the market a high-growth, innovation-driven global healthcare segment.
Pulmonary Edema Market Set to Skyrocket as Cardiovascular Diseases and Trauma Surge – Advanced Treatments and Diagnostics Drive Explosive Growth
Pulmonary Edema Market is set to surge as the global prevalence of cardiovascular diseases (CVDs) skyrockets. Heart attacks, strokes, and hypertension contribute to over 16.8 million deaths annually worldwide, driving urgent demand for effective pulmonary edema management and treatment solutions. Adding to the challenge, road accidents causing chest injuries claim 1.19 million lives each year, particularly among young adults, often leading to acute pulmonary complications. This growing healthcare burden is pushing hospitals, clinics, and home care providers to adopt advanced diagnostics, oxygen therapy, mechanical ventilation, and novel treatment options, creating dynamic growth opportunities in the Pulmonary Edema Market.
👉 Access the full Research Description at:
https://www.stellarmr.com/report/req_sample/pulmonary-edema-market/2673
Pulmonary Edema Market Poised for Explosive Growth as Aging Population Drives Surge in Heart, Kidney, and Cardiovascular Cases
Pulmonary Edema Market is poised for rapid growth as the global population ages, driving higher incidence of heart, kidney, and cardiovascular conditions, key risk factors for pulmonary edema. With age-related diseases on the rise, healthcare systems face growing demand for advanced pulmonary edema treatments, diagnostics, and management solutions. This demographic shift presents a massive growth opportunity, positioning the Pulmonary Edema Market as one of the most dynamic and fast-evolving segments in global healthcare.
Pulmonary Edema Market Faces High-Stakes Challenges:
Rising Costs, Access Gaps, and Treatment Risks Spark Urgent Need for Innovation
Pulmonary Edema Market is expanding rapidly, but significant challenges and risks remain. High treatment costs, limited access to advanced diagnostics and therapies in developing regions, and potential side effects of medications could hinder market growth. Additionally, disparities in healthcare infrastructure and regulatory hurdles pose obstacles for timely patient care. To navigate these risks, stakeholders are advised to invest in telemedicine, remote monitoring solutions, and innovative treatment protocols, ensuring improved
patient outcomes while capitalizing on the market’s growth potential.
Pulmonary Edema Market Set to Soar:
Cardiogenic and Non-Cardiogenic Cases Drive Demand for Advanced Diagnostics and Innovative Treatments
Pulmonary Edema Market is witnessing rapid growth across disease types, diagnostics, and treatments. Cardiogenic pulmonary edema, linked to heart failure, coronary artery disease, and cardiomyopathy, dominates, affecting millions in the U.S. with high mortality, while non-cardiogenic pulmonary edema, caused by lung injury, infections, trauma, or high-altitude exposure, drives demand for oxygen therapy, mechanical ventilation, and innovative medications. Advanced diagnostics, including chest X-rays, CT scans, and AI-assisted lung ultrasounds, enable early detection, while hospitals, clinics, and ambulatory surgical centers provide critical care.
Key Pulmonary Edema Market Trends:
Rising Risk Factors and Growth Opportunities in Emerging Economies
Increasing Prevalence of Underlying Conditions: A growing incidence of heart failure, cardiovascular diseases, and chronic lung disorders is driving expansion in the Pulmonary Edema Market, boosting demand for advanced treatments and diagnostics.
Market Expansion in Emerging Economies: Rising health insurance coverage in countries like China and India is enhancing patient access to pulmonary edema treatments, driving significant market growth.
Key Developments in the Pulmonary Edema Market:
Strategic Acquisitions and Innovative Collaborations
ResMed and Propeller Health Acquisition: In April 2023, ResMed acquired Propeller Health, boosting remote monitoring and data-driven care for pulmonary edema.
AstraZeneca and BioNTech Collaboration: In March 2023, AstraZeneca partnered with BioNTech to develop novel therapies targeting pulmonary edema linked to lung cancer.
👉 Access the full Research Description at:
https://www.stellarmr.com/report/req_sample/pulmonary-edema-market/2673
North America Dominates Pulmonary Edema Market:
Rising Hospitalizations and High Mortality Highlight Urgent Need for Advanced Treatments
Pulmonary Edema Market is dominated by North America, driven by a well-developed healthcare sector and high per capita spending. Acute cardiogenic pulmonary edema accounts for 1 million hospital admissions annually in the U.S., with high mortality rates, while England and Wales report over 67,000 admissions from acute heart failure. This significant healthcare burden underscores the urgent need for advanced treatment strategies, effective management, and improved patient outcomes, highlighting key growth opportunities in diagnostics and therapeutics.
Pulmonary Edema Market Soars:
Pfizer, Novartis, and Verve Drive Innovation with FDA Approvals and Cutting-Edge Gene Therapies
Leading companies like Pfizer, Novartis, Medtronic, Siemens Healthineers, and BD are advancing pulmonary edema treatments through innovative therapies and clinical trials. Novartis secured FDA approval for Entresto targeting heart failure with preserved ejection fraction (HFpEF), while Verve Therapeutics initiated Phase 1b trials for VERVE-101, a promising gene therapy for pulmonary edema. These developments highlight a surge in cutting-edge treatments and market opportunities, offering hope for improved patient outcomes and reduced mortality.
Key Players in the Pulmonary Edema Market
North America
Pfizer Inc. (USA)
ARGON MEDICAL (USA)
Edwards Lifesciences Corporation (USA)
BD (Becton, Dickinson and Company) (USA)
Abbott Laboratories (USA)
Johnson & Johnson (USA)
Merck & Co. (USA)
GE Healthcare (USA)
ResMed (USA)
United Therapeutics Corporation (USA)
Boehringer Laboratories (USA)
Medtronic (USA)
AstraZeneca (UK)
Europe
Bayer AG (Germany)
Boehringer Ingelheim (Germany)
Siemens Healthcare GmbH (Germany)
Fresenius Medical Care (Germany)
Novartis AG (Switzerland)
Roche Holding AG (Switzerland)
Actelion Pharmaceuticals Ltd. (Switzerland)
Sanofi S.A. (France)
Chiesi Farmaceutici (Italy)
Koninklijke Philips N.V. (Netherlands)
GlaxoSmithKline plc (UK)
Asia Pacific
Lupin Pharmaceuticals (India)
Hitachi Medical Corporation (Japan)
Canon Medical Systems Corporation (Japan)
Vitaltec Corporation (Taiwan)
Analyst Perspective:
Global Pulmonary Edema Market is set for strong growth, driven by rising cardiovascular diseases, aging populations, and trauma cases. North America leads, while China and India offer growth opportunities. Key players like Pfizer, Novartis, ResMed, AstraZeneca, and Medtronic are expanding the market via FDA approvals, innovative therapies, acquisitions, and collaborations. Advanced diagnostics, AI imaging, oxygen therapy, and gene therapies are enhancing outcomes, positioning pulmonary edema as a high-growth, innovation-driven healthcare segment.
FAQ:
Q1: What is the Pulmonary Edema Market size and growth forecast?
A1: The global Pulmonary Edema Market is valued at USD 6.6 Bn in 2024 and projected to reach USD 12.04 Bn by 2032 at a CAGR of 7.8%.
Q2: What are the key drivers of Pulmonary Edema Market growth?
A2: Rising cardiovascular diseases, heart failure, chronic lung disorders, trauma-related injuries, and aging populations are driving market expansion.
Q3: Who are the major players in the Pulmonary Edema Market?
A3: Leading companies include Pfizer, Novartis, ResMed, AstraZeneca, Medtronic, Siemens Healthineers, and BD, driving innovation through FDA approvals, gene therapies, and strategic collaborations.
Maximize Market Research is launching a subscription model for data and analysis in the
Dental Materials market
https://www.mmrstatistics.com/markets/512/topic/833/pharmaceuticals
Related Reports:
Pulmonary Edema Market:
https://www.stellarmr.com/report/pulmonary-edema-market/2673
Ureteral Obstruction Market:
https://www.stellarmr.com/report/ureteral-obstruction-market/2614
Pleurisy Market:
https://www.stellarmr.com/report/pleurisy-market/2612
Anti-aging Cosmetics Market:
https://www.stellarmr.com/report/anti-aging-cosmetics-market/2562
Continence Care Products Market:
https://www.stellarmr.com/report/continence-care-products-market/2483
About Stellar Market Research:
Stellar Market Research is a multifaceted market research and consulting company with professionals from several industries. Some of the industries we cover include medical devices, pharmaceutical manufacturers, science and engineering, electronic components, industrial equipment, technology and communication, cars and automobiles, chemical products and substances, general merchandise, beverages, personal care, and automated systems. To mention a few, we provide market-verified industry estimations, technical trend analysis, crucial market research, strategic advice, competition analysis, production and demand analysis, and client impact studies.
Lumawant Godage
Stellar Market Research
+ +91 9607365656
email us here
Visit us on social media:
LinkedIn
Instagram
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability
for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this
article. If you have any complaints or copyright issues related to this article, kindly contact the author above.