Request Demo
Last update 13 Dec 2025
Alicaforsen sodium
Last update 13 Dec 2025
Overview
Basic Info
Drug Type ASO |
Synonyms Alicaforsen, Alicaforsen enema, Alicaforsen sodium (USAN) + [11] |
Target |
Action inhibitors |
Mechanism ICAM-1 inhibitors(Intercellular adhesion molecule-1 inhibitors), Immunosuppressants |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
License Organization |
Drug Highest PhaseDiscontinuedPhase 3 |
First Approval Date- |
RegulationOrphan Drug (United States) |
Login to view timeline
Structure/Sequence
Boost your research with our RNA technology data.
login
or
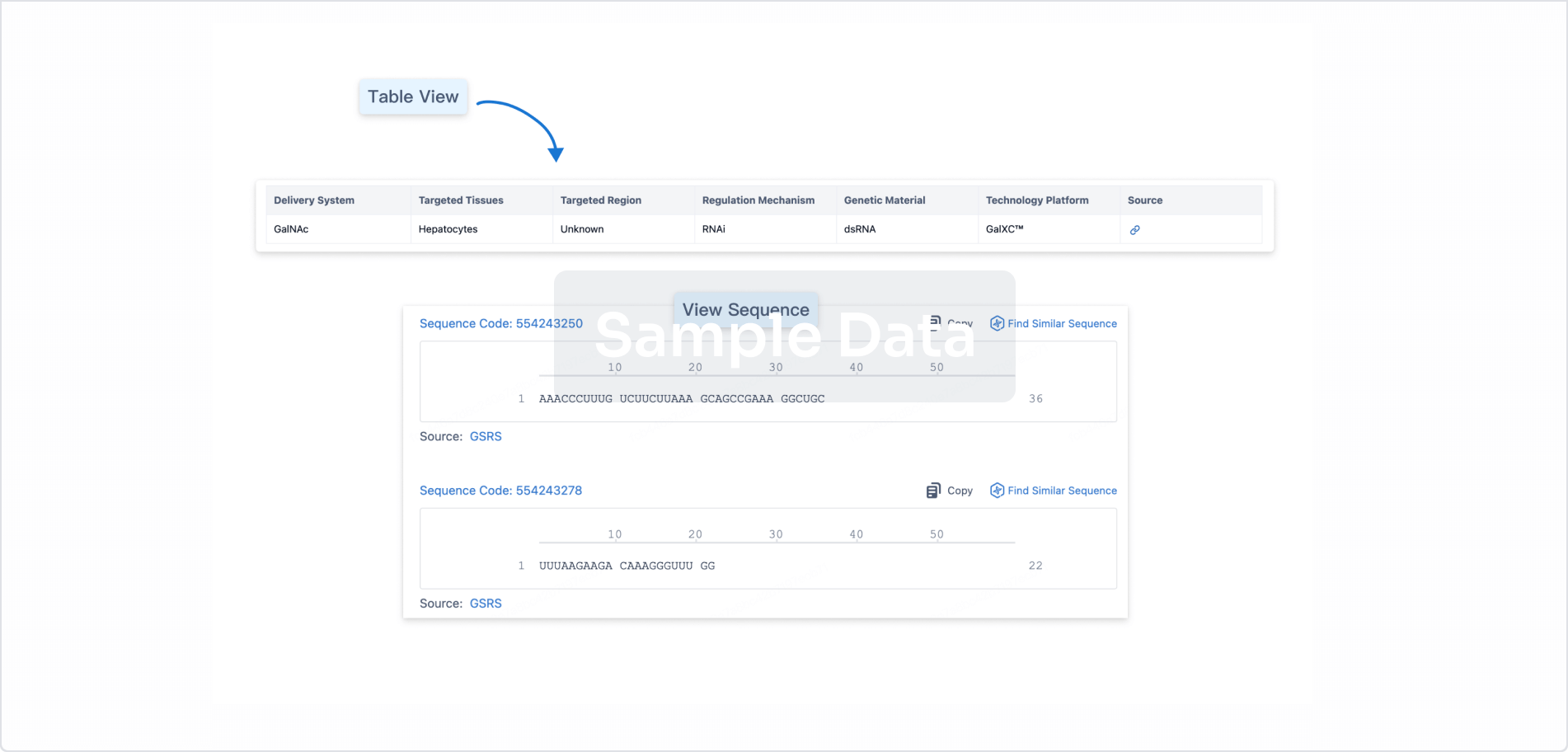
Sequence Code 29268246

Source: *****
Related
6
Clinical Trials associated with Alicaforsen sodiumNCT03473626
A Two-Period, Randomised, Crossover Study Designed to Evaluate the In Vivo Performance of a Modified Release Formulation of Alicaforsen in Healthy Male Subjects in the Fed and Fasted State
Phase 1 study to compare the effect of food on alicaforsen tablet.
Start Date16 Mar 2018 |
Sponsor / Collaborator |
NCT02525523
A Randomized, Double-Blind, Placebo-Controlled Trial of the Safety and Efficacy of Topical Alicaforsen Enema in Subjects With Active, Chronic, Antibiotic Refractory Primary Idiopathic Pouchitis
A Phase III, multi-centre, double-blind randomised controlled trial in subjects with chronic antibiotic refractory pouchitis.
Subjects will undertake a <2 week screening period to provide baseline data and be assessed for eligibility. At the Baseline visit (Day 1) eligible subjects will be randomised on a 1:1 basis to either a) 240 mg alicaforsen enema or b) matching placebo.
Study drug will be administered once nightly (on going to bed) up to and including week 6. Following the Day 1 Visit, subjects will return to the clinic for safety and efficacy assessments at Week 3, 6, 10, 18 and 26.
Subjects may receive certain permitted medications as per Entry Criteria, which must remain at stable doses throughout the trial. Introduction of any new medication for pouchitis, or a dose change to an existing concomitant medication for pouchitis, other than those detailed in the protocol, will not be permitted.
Clinical symptoms associated with pouchitis will be recorded daily by the patient in a diary card.
Subjects will undergo endoscopic examination of their pouch (during Screening, and at Weeks 6 and 10). Where technically feasible, each endoscopy will provide at least one biopsy sample for histopathology.
In addition to endoscopic, histopathologic and symptomatic assessments, Quality of Life will be assessed.
Bloods for routine assessment, including haematology and biochemistry will be taken. Bloods and stool samples will be collected to evaluate relevant biomarkers.
Subjects will undertake a <2 week screening period to provide baseline data and be assessed for eligibility. At the Baseline visit (Day 1) eligible subjects will be randomised on a 1:1 basis to either a) 240 mg alicaforsen enema or b) matching placebo.
Study drug will be administered once nightly (on going to bed) up to and including week 6. Following the Day 1 Visit, subjects will return to the clinic for safety and efficacy assessments at Week 3, 6, 10, 18 and 26.
Subjects may receive certain permitted medications as per Entry Criteria, which must remain at stable doses throughout the trial. Introduction of any new medication for pouchitis, or a dose change to an existing concomitant medication for pouchitis, other than those detailed in the protocol, will not be permitted.
Clinical symptoms associated with pouchitis will be recorded daily by the patient in a diary card.
Subjects will undergo endoscopic examination of their pouch (during Screening, and at Weeks 6 and 10). Where technically feasible, each endoscopy will provide at least one biopsy sample for histopathology.
In addition to endoscopic, histopathologic and symptomatic assessments, Quality of Life will be assessed.
Bloods for routine assessment, including haematology and biochemistry will be taken. Bloods and stool samples will be collected to evaluate relevant biomarkers.
Start Date03 Dec 2015 |
Sponsor / Collaborator |
NCT00063830
ISIS 2302-CS27, Phase 2, Double-Blinded, Controlled Study of Four Dosing Regimens of Alicaforsen (ISIS 2302) Enema, an Antisense Inhibitor of ICAM-1, for the Treatment of Patients With Mild to Moderate Active Ulcerative Colitis
This is a multi-center trial in the US and Europe to test the safety, efficacy and tolerability of alicaforsen (ISIS 2302), a new type of drug called an antisense drug, in patients with mild to moderate active Ulcerative Colitis (UC). Alicaforsen is designed to reduce the production of a specific protein, called ICAM-1, a substance that plays a significant role in the increase of inflammation and is likely to be involved in inflammatory bowel diseases such as ulcerative colitis. The ISIS 2302-CS27 study will compare four dosing regimens and determine the minimum effective dose of alicaforsen enema in UC patients over six weeks as compared to a placebo enema. (The probability of receiving active formulation is 4:1). The primary objective of this study is to evaluate the percentage reduction in DAI at Week 6.
Start Date03 Apr 2003 |
Sponsor / Collaborator |
100 Clinical Results associated with Alicaforsen sodium
Login to view more data
100 Translational Medicine associated with Alicaforsen sodium
Login to view more data
100 Patents (Medical) associated with Alicaforsen sodium
Login to view more data
44
Literatures (Medical) associated with Alicaforsen sodium03 Oct 2025·NUCLEOSIDES NUCLEOTIDES & NUCLEIC ACIDS
Bioinformatics analysis of key genes and potential therapeutic agents for vascular calcification in chronic kidney disease
Article
Author: Liu, Tengfei ; Ding, Chunhua ; Ye, Guojie
Vascular calcification is a common complication of chronic kidney disease (CKD). The molecular mechanisms underlying this condition and the efficacy of potential treatments remain unclear. Bioinformatic methods were employed to analyze gene ontology (GO) annotations and pathway enrichments. Subsequently, an analysis of potential therapeutic agents for vascular calcification in CKD was performed. A total of 76 common genes, 181 enriched GO annotations-comprising 153 biological processes, 10 cellular components, and 18 molecular functions-41 KEGG pathways, 13 REACTOME pathways, and 3 BIOCARTA pathways were identified. Five key genes (PSMC5, TNFSF11, TNFRSF11A, TNFRSF12A, and ICAM1) were isolated. Most notably, the top five potential therapeutic drugs-ENAVATUZUMAB, DENOSUMAB, ALICAFORSEN, BI-505, and ENLIMOMAB PEGOL-were identified for vascular calcification in CKD. However, further molecular biological experiments are required to confirm these findings.
01 Jul 2025·Clinical Gastroenterology and Hepatology
Development and External Validation of an Endoscopic and Histologic Pouchitis Assessment Tool: The Atlantic Pouchitis Index
Article
Author: Travis, Simon ; Samaan, Mark A ; Panaccione, Remo ; McFarlane, Stefanie C ; Rémillard, Julie ; Silverberg, Mark S ; Hogan, Malcolm ; Ma, Christopher ; Marchal, Aude ; Dotan, Iris ; Hart, Ailsa ; Bojic, Bozidar ; Rosenfeld, Gregory ; Kirsch, Richard ; Sands, Bruce E ; Feagan, Brian G ; D'Haens, Geert R ; Feakins, Roger M ; Zou, Guangyong ; Sedano, Rocio ; Bressler, Brian ; Jairath, Vipul ; Shackelton, Lisa M
BACKGROUND & AIMS:
No fully validated indices to measure pouchitis activity exist. We aimed to develop and externally validate a novel endoscopic and histologic index.
METHODS:
Endoscopists and pathologists used 11 (4 endoscopic, 4 histologic, 3 composite) existing indices and items from a prior Research and Development/University of California Los Angeles appropriateness exercise to assess pouchitis disease activity in videos and images from 98 patients with chronic antibiotic-refractory pouchitis who participated in a randomized placebo-controlled alicaforsen trial. Reliability was assessed with the intraclass correlation coefficient (ICC). Responsiveness was quantified by the area under the receiver operating characteristic curve (AUROC). A novel index was developed using linear regression with a visual analog scale (VAS) of pouchitis endoscopic and histologic disease activity as the dependent variable and was externally validated with the EARNEST vedolizumab trial data.
RESULTS:
The Atlantic Pouchitis Index (API) (range, 0-69) comprises the Simple Endoscopic Score for Crohn's Disease and Robarts Histopathology Index. The API exhibited almost perfect intra-rater (ICC, 0.88; 95% confidence interval [CI], 0.81-0.92) and substantial inter-rater reliability (ICC, 0.72; 95% CI, 0.60-0.79). A high degree of responsiveness (AUROC, 0.95; 95% CI, 0.89-0.98), greater than existing endoscopic indices (ΔAUROC, 0.09-0.24; P ≤ .005), was observed when the change criterion was a decrease in the VAS of one-half of the standard deviation. Good responsiveness was observed in external validation when vedolizumab was the change criterion (AUROC, 0.63; 95% CI, 0.51-0.73).
CONCLUSION:
Development and validation of the API advances pouchitis disease assessment. Integration of endoscopy and histopathology results in an objective, reliable, and responsive instrument to evaluate therapy effectiveness in research and clinical settings.
01 Feb 2019·Best practice & research. Clinical gastroenterologyQ3 · MEDICINE
Interfering with leukocyte trafficking in Crohn's disease
Q3 · MEDICINE
Review
Author: Travis, Simon ; Bryant, Robert V ; Biswas, Sujata
The discovery of gut-specific leukocytes and the ability to modulate their function has been a groundbreaking development in the treatment of inflammatory bowel disease. Drugs target the interaction between lymphocytes and endothelial cells via integrins and their ligand cellular adhesion molecules. Safety, efficacy and sustainability of effect are key to this drug class, notwithstanding the association of natalizumab with fatal polyoma virus infection. Vedolizumab (2014) now licensed for the treatment of Crohn's disease around the world provides gut-specific immunosuppression. Targets for modulators of leukocyte trafficking include (examples in brackets) ICAM-1 (alicaforsen, efalizumab); MAdCAM-1 (PF-00547 659); α4 and related receptors (abrilumab, etrolizumab, natalizumab, vedolizumab); chemokine receptor CCR9 (vercirnon); and sphingosine 1-phosphate receptors (etrasimod, fingolimod, ozanimod). Oral and subcutaneous therapies are in development. The safety, efficacy and practice points of licensed drugs are discussed, in addition to initial results from therapeutic trials.
3
News (Medical) associated with Alicaforsen sodium31 Jul 2019
Atlantic Healthcare plc, a specialist pharmaceutical company focused on acquiring, developing and commercializing therapeutics that address unmet patient needs and rare diseases, announced the results of the Phase 3 trial of Camligo™ for orphan-designated pouchitis.
Cambridge, UK; July 31, 2019.
Atlantic Healthcare plc
(“Atlantic Healthcare” or “Company”), a specialist pharmaceutical company focused on acquiring, developing and commercializing therapeutics that address unmet patient needs and rare diseases, today announced the results of the Phase 3 trial of Camligo™ (alicaforsen enema) for orphan-designated pouchitis.
In the primary analysis, using an adaptation of the Mayo Score of improvement in endoscopic remission and bowel frequency, the trial did not meet its co-primary endpoints.
However, the data for stool frequency do show an encouraging efficacy signal and remission in 34% of patients. In addition, a re-evaluation of the endoscopy data, using new methods of analysis
[1]
, indicates a statistically significant endoscopic response was achieved in a number of subgroups of patients.
Toby Wilson Waterworth, Chief Executive, Atlantic Healthcare plc said: “Although we are disappointed that the Phase 3 trial did not achieve statistical significance, we believe the percentage of patients achieving remission in stool frequency and the endoscopic response observed in a number of sub populations of patients could be noteworthy. Having consulted with key opinion leaders and regulatory advisors, we now plan to meet with the U.S. Food and Drug Administration and European Medicines Agency to discuss a pathway to regulatory approval.”
He added, “We are grateful to the clinicians and patients who participated in the trial, which confirmed that alicaforsen is safe and well-tolerated, with no drug-related serious adverse events. It is also encouraging to note that the patients who participated in the trial demonstrated strong compliance with alicaforsen enema.”
Prof Brian Feagan MD, FRCPC, an acknowledged expert in IBD and Director at Robarts Research Institute added: “It is challenging to develop treatments for orphan-designated diseases. This study makes a significant contribution to the existing body of knowledge in pouchitis. As long as there is no approved treatment, there remains an unmet medical need. It is therefore encouraging to note that alicaforsen enema may benefit some pouchitis patients.”
About pouchitis
Pouchitis is estimated to impact the lives of approximately 200,000 patients in the U.S. and Europe. It is a progressive disease characterized by inflammation, ulceration and increasingly uncontrolled, frequent and urgent emptying of the bowel (up to 10-20 times a day and night).
Camligo™ (alicaforsen enema) Phase 3 Trial
Camligo™ is the brand name of alicaforsen enema to treat orphan-designated pouchitis. The trial was a Phase 3, multi-center, double-blind randomized controlled trial in subjects with chronic antibiotic refractory pouchitis (failed to adequately respond to one or more courses of antibiotics). The trial recruited 138 patients at 40 centers across the U.S., Canada and Europe. Patients received Camligo™ 240mg once daily for six weeks, or placebo. The primary endpoints of the trial were the proportion of patients achieving endoscopic remission and a reduction in stool frequency at week 10. Secondary endpoints assessed improvement in Quality of Life.
About Atlantic Healthcare
Atlantic Healthcare plc is focused on acquiring, developing and commercializing therapeutics that can address unmet needs of patients who are managed by healthcare professionals in hospital and specialist care environments. The Company owns the exclusive worldwide rights to alicaforsen, a novel antisense drug. Recently, it also acquired the global rights to renzapride, which has the potential to address the unmet needs of multiple patient groups with gastrointestinal motility disorders.
Atlantic Healthcare intends to commercialize its products in Europe and the U.S. using a specialist sales team targeting healthcare professionals based in hospitals and specialist care centers. The Company plans to partner with established pharmaceutical companies to commercialize its products in the rest of the world.
The Company is led by an experienced international Board and Leadership Team, with deep roots and a proven track record in the pharmaceutical industry.
Alicaforsen
Alicaforsen is an antisense oligonucleotide with the potential to treat multiple patient groups experiencing inflammatory diseases of the GI tract, including ulcerative colitis (UC) and Crohn’s Disease. Atlantic Healthcare is developing and deepening the pipeline with plans for a Phase 3 trial of alicaforsen enema for distal colitis and Phase 2b trials in alicaforsen tablet for UC and Crohn’s.
For more information about alicaforsen and how it works, please go to:
com/research/alicaforsen/
For published papers please go to:
com/research/publications/
Forward Looking Statements
This press release contains forward looking statements that are based on management’s beliefs and assumptions as of the date of this press release. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from those indicated by such statements. These forward-looking statements involve risks and uncertainties, including, among others, that the development of any products for their stated uses may not proceed due to safety, efficacy or other reasons. In addition, risks and results in clinical trials may not be indicative of risks or results from later stage or larger scale trials, and there is no assurance of regulatory approval. Existing and prospective investors should not place undue reliance on the forward-looking statements contained in this press release and instead should make their own determinations as to the reliability of such statements. Atlantic Healthcare undertakes no intent or obligation to update the information contained in this press release.
For more information please contact:
Atlantic Healthcare
Adam Michael
+44 1799 512 055
+44 777 588 1813
adam.michael@atlantichc.com
U.S. Investor Relations and Media
Lazar Partners
David Carey / Amy Feldman
+1 212-867-1762
atlantic.healthcare@
lazarpartners.com
European Investor Relations and Media
Consilium Strategic Communications
Mary-Jane Elliott / Matthew Neal
+44 20 3709 5700
atlantichealthcare@consilium-
comms.com
[1] Samaan et al “Reliability among central readers in the evaluation of endoscopic disease activity in pouchitis”, American Society for Gastroenterology Endoscopy, Sept 2018
Clinical ResultPhase 3Orphan DrugAcquisitionPhase 2
30 Sep 2018
Oral alicaforsen appears safe and well-tolerated
Oral alicaforsen appears safe and well-tolerated
Cambridge, UK; 27 September 2018 --
Atlantic Healthcare
plc
(“Atlantic Healthcare” or “Company”), a specialist pharmaceutical company focused on developing and commercializing gastrointestinal therapeutics, announces that its Phase 1 trial of an oral alicaforsen tablet for Crohn’s disease found that the formulation appears to be safe and well-tolerated.
The trial was designed to evaluate safety and tolerability and confirm the site of release of the oral alicaforsen tablet, for delivery to the small intestine, where Crohn’s disease is most prevalent. A total of 15 healthy male volunteers received 240mg alicaforsen in oral tablet form on two separate occasions, once with food and once fasted.
The results demonstrated that the oral tablet appeared to deliver product safely, with all tablets disintegrating to release product before the end of the small intestine. In addition, in line with expectations, the trial showed minimal systemic absorption following oral administration, which is desirable for targeted topical activity within the gut.
Atlantic Healthcare will now start planning for a Phase 2b efficacy trial of alicaforsen tablet in Crohn’s.
Toby Wilson Waterworth, CEO, Atlantic Healthcare commented:
“We are delighted to have shown that alicaforsen appears to be delivered safely to the small intestine in oral tablet form, and that it is well tolerated. When viewed alongside results from other alicaforsen clinical trials in ulcerative colitis, the minimal systemic absorption reinforces the drug’s potential as a topical product for Inflammatory Bowel Disease, whether administered orally or rectally.”
Alicaforsen has the potential to create a new class of treatment for Inflammatory Bowel Disease (IBD) and other inflammatory diseases. Atlantic Healthcare’s lead program, Camligo™ (alicaforsen) enema for the treatment of pouchitis, is in a Phase 3 trial, with results expected in Q1 2019.
For more information about Atlantic Healthcare and the alicaforsen pipeline, please go to
About Atlantic Healthcare plc (
)
“Atlantic Healthcare” is a specialist pharmaceutical company focused on developing and commercializing gastrointestinal therapeutics, which address unmet patient needs and rare diseases. The Company owns the exclusive worldwide rights to alicaforsen, a promising new drug for the treatment of inflammation. Its most advanced development program – Camligo™ (alicaforsen) enema - is undergoing a pivotal Phase 3 trial for pouchitis. In addition, work is ongoing to develop and deepen the pipeline for alicaforsen with treatments for Crohn’s disease, ulcerative colitis and other gastrointestinal (GI) indications.
Atlantic Healthcare has a highly committed investor base and is led by an experienced international Board and Leadership Team, with deep roots and a proven track record in the pharmaceutical industry. Fundraising to date includes £1.9m through SBRI funding awarded by Innovate UK, the UK Government’s innovation agency (
). In Q1 2016 the Company closed a $24m round led by LDC (the private equity division of Lloyd’s Banking Group) with new and existing investors.
Crohn’s disease
Crohn’s Disease is an
inflammatory disease which can affect any part of the digestive system, from the mouth to the anus. However, it most commonly occurs in the last section of the small intestine (ileum). Common symptoms can include diarrhoea, abdominal pain and the presence of blood and mucus in the stool. Over time, inflammation can damage sections of the GI tract, resulting in complications which usually require surgery to remove the diseased tissue.
In 2016, there were estimated to be 1.3m patients diagnosed with Crohn’s in the U.S. and European markets (Source: Decision Resources).
About Alicaforsen
Alicaforsen is a novel antisense drug. It is a pipeline in a drug, with the potential to establish a new class of therapy for the treatment of multiple inflammatory gastrointestinal disorders.
For more information about alicaforsen and how it works, please go to:
For published papers please go to:
papers
Forward Looking Statements
This press release contains forward-looking statements including, without limitation, the information provided regarding future Phase 2b efficacy trials, the potential use of alicaforsen as a topical product for Inflammatory Bowel Disease (“IBD”), the potential to create a new class of treatment and use for IBD and other inflammatory diseases, and the expectation of obtaining Phase 3 results in 2019 for Camligo™ (alicaforsen). While Atlantic Healthcare believes the forward-looking statements in this press release are accurate, the statements represent Atlantic Healthcare’s beliefs only as of the date of this press release. A number of factors could cause actual events or results to differ materially from those indicated by such statements. These forward-looking statements involve risks and uncertainties, including, among others, that the development of alicaforsen for the stated uses may not proceed due to safety, efficacy or other reasons. In addition, risks and results in clinical trials may not be indicative of risks or results from later stage or larger scale trials, and there is no assurance of regulatory approval. Existing and prospective investors should not place undue reliance on the forward-looking statements contained in this press release and instead should make their own determinations as to the reliability of such statements. Atlantic Healthcare undertakes no intent or obligation to update the information contained in this press release as new information becomes available.
For information please contact:
Atlantic Healthcare
Adam Michael (Head of Communications)
+44 1799 512 055
+44 777 588 1813
adam.michael@atlantichc.com
U.S. Investor Relations and Media
Lazar Partners
Fern Lazar / David Carey
+1 212-867-1762
atlantic.healthcare@
lazarpartners.com
European Investor Relations and Media
Consilium Strategic Communications
Mary-Jane Elliott / Matthew Neal
+44 20 3709 5700
atlantichealthcare@consilium-
comms.com
Phase 3Phase 1Clinical ResultPhase 2
14 Sep 2018
Atlantic Healthcare plc has secured acceptance of Camligo™ as the name for the Company’s first candidate product.
Cambridge, UK; September 13, 2018 --
Atlantic Healthcare
plc
(“Atlantic Healthcare” or “Company”), a specialist pharmaceutical company focused on developing and commercializing gastrointestinal therapeutics, has secured acceptance of Camligo™ as the name for the Company’s first candidate product.
The U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA), have accepted Camligo™ as the proprietary brand name for alicaforsen enema, which is currently undergoing a pivotal Phase 3 trial in the treatment of orphan-designated pouchitis.
Pouchitis is a rare and serious form of Inflammatory Bowel Disease (IBD), which is believed to impact the lives of approximately 200,000 patients in the U.S and Europe. There is no approved treatment for pouchitis, hence there remains a significant unmet clinical need.
In May 2017 Atlantic Healthcare initiated the rolling submission of its New Drug Application (NDA) with the U.S. Food and Drug Administration (FDA), and filed its non-clinical package for alicaforsen to treat pouchitis.
John Temperato, U.S. President and COO at Atlantic Healthcare said:
“We are acutely aware of the unmet needs of pouchitis patients and the debilitating effects the disease can have on their quality of life. Now this brand name has been agreed with the U.S. and European regulatory authorities, we have met another critical milestone towards Camligo™ (alicaforsen) enema being available as a treatment for these patients.”
Toby Wilson Waterworth, CEO at Atlantic Healthcare said:
“We look forward to announcing the results of the Phase 3 trial in the first quarter of 2019 and being able to complete regulatory submissions for Camligo™ with the FDA and EMA. Following approval Camligo™ would be the first and only approved treatment for pouchitis. In the meantime, we continue to broaden the commercial potential and develop the pipeline of alicaforsen products for the treatment of ulcerative colitis, Crohn’s disease and other inflammatory diseases.”
For more information about Camligo™ (alicaforsen) enema for pouchitis and the alicaforsen pipeline, please see
.
For information please contact:
Atlantic Healthcare
Toby Wilson Waterworth (CEO)
+44 1799 512 055
Adam Michael (Head of Communications)
+44 1799 512 055
+44 777 588 1813
adam.michael@atlantichc.com
U.S. Investor Relations and Media
Lazar Partners
Fern Lazar / David Carey
+1 212-867-1762
atlantic.healthcare@
lazarpartners.com
European Investor Relations and Media
Consilium Strategic Communications
Mary-Jane Elliott / Matthew Neal
+44 20 3709 5700
atlantichealthcare@consilium-
comms.com
NOTES TO EDITORS
About Atlantic Healthcare plc (
)
“Atlantic Healthcare” is a specialist pharmaceutical company focused on developing and commercializing gastrointestinal therapeutics, which address unmet patient needs and rare diseases. The Company owns the exclusive worldwide rights to alicaforsen, a promising new drug for the treatment of inflammation. Its most advanced development program – alicaforsen enema - is undergoing a pivotal Phase 3 trial for pouchitis. In addition, work is ongoing to develop and deepen the pipeline for alicaforsen with treatments for additional gastrointestinal (GI) indications.
Atlantic Healthcare has a highly committed investor base and is led by an experienced international Board and Leadership Team, with deep roots and a proven track record in the pharmaceutical industry. Fundraising to date includes £1.9m through SBRI funding awarded by Innovate UK, the UK Government’s innovation agency (
). In Q1 2016 the Company closed a $24m round led by LDC (the private equity division of Lloyd’s Banking Group) with new and existing investors.
About Pouchitis
Pouchitis is an inflammatory disease of the pouch. The pouch is an artificial rectum, which is created surgically following removal of the colon (“colectomy”). Indications for removal of the colon include ulcerative colitis, colon cancer and FAP (“familial adenomatous polyposis”, a pre-cancerous condition). However, pouchitis is predominantly associated with pouches formed in patients with a prior diagnosis of ulcerative colitis.
Like ulcerative colitis, pouchitis is a progressive disease characterised by inflammation, ulceration and increasingly uncontrolled, frequent and urgent emptying of the bowel (up to 10-20 times a day and night), with a corresponding reduction in quality of life.
For information about prevalence, treatment and the unmet needs of pouchitis patients please see
inflammatory-bowel-disease
.
About Camligo™ (alicaforsen) enema
Camligo™ alicaforsen enema formulation is currently in a pivotal Phase 3 trial agreed with U.S., Canadian and European regulatory agencies in patients with active pouchitis. The trial has completed enrollment of 138 patients to approximately 40 trial centers across the U.S., Canada, Europe, and Israel, and is expected to report preliminary data in Q1 2019.
Trials have shown Camligo™ (alicaforsen) enema to be a safe, well tolerated and effective treatment, which delivers a durable response for pouchitis patients.
About the Camligo™ name
Commenting on the name, the US Food and Drug Association (FDA) “concluded that it is conditionally acceptable.”
The European Medicine Agency (EMA) said: “The Name Review Group (NRG) and The Committee for Medicinal Products for Human Use (CHMP) have no objections to the invented name Camligo. It should be noted, the (invented) name review is valid, at the present point in time, which does not prohibit the possibility of objections being raised at any time prior or after the granting of the marketing authorization.”
The Camligo™ trademark has been registered in the UK and is pending in Europe and the U.S..
About Alicaforsen
Alicaforsen is a novel antisense drug. It is a pipeline in a drug, with the potential to establish a new class of therapy for the treatment of multiple inflammatory gastrointestinal disorders.
For further reading:
papers
Phase 3NDADrug Approval
100 Deals associated with Alicaforsen sodium
Login to view more data
External Link
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| D02811 | Alicaforsen sodium | - |
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Pouchitis | Phase 3 | United States | 03 Dec 2015 | |
| Pouchitis | Phase 3 | Belgium | 03 Dec 2015 | |
| Pouchitis | Phase 3 | Canada | 03 Dec 2015 | |
| Pouchitis | Phase 3 | France | 03 Dec 2015 | |
| Pouchitis | Phase 3 | Ireland | 03 Dec 2015 | |
| Pouchitis | Phase 3 | Israel | 03 Dec 2015 | |
| Pouchitis | Phase 3 | Italy | 03 Dec 2015 | |
| Pouchitis | Phase 3 | Netherlands | 03 Dec 2015 | |
| Pouchitis | Phase 3 | Switzerland | 03 Dec 2015 | |
| Pouchitis | Phase 3 | United Kingdom | 03 Dec 2015 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 3 | 138 | puuodconrr(ctbessdiyi) = cyqquvyqya ihhkjjcike (vekuvvgfko ) View more | Positive | 02 Oct 2021 | |||
Placebo enema | puuodconrr(ctbessdiyi) = ricikqxvvg ihhkjjcike (vekuvvgfko ) View more | ||||||
Phase 3 | 138 | (Alicaforsen) | jhigcvuwyw = ehyyvkidtx kqtwjunzpg (gbcydatyoy, hthzavrtky - amnjxesluz) View more | - | 25 Feb 2020 | ||
Placebo (Placebo) | jhigcvuwyw = jblczxhwyp kqtwjunzpg (gbcydatyoy, eawgzolczg - zemefwcsik) View more |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free

