Request Demo
Last update 30 Jul 2025
TQB-2102
Last update 30 Jul 2025
Overview
Basic Info
Drug Type Antibody drug conjugate (ADC) |
Synonyms TQB 2102, TQB-2102 |
Target |
Action antagonists, inhibitors |
Mechanism HER2 antagonists(Receptor tyrosine-protein kinase erbB-2 antagonists), TOP1 inhibitors(DNA topoisomerase I inhibitors) |
Therapeutic Areas |
Inactive Indication- |
Originator Organization |
Inactive Organization- |
License Organization- |
Drug Highest PhasePhase 3 |
First Approval Date- |
RegulationBreakthrough Therapy (China) |
Login to view timeline
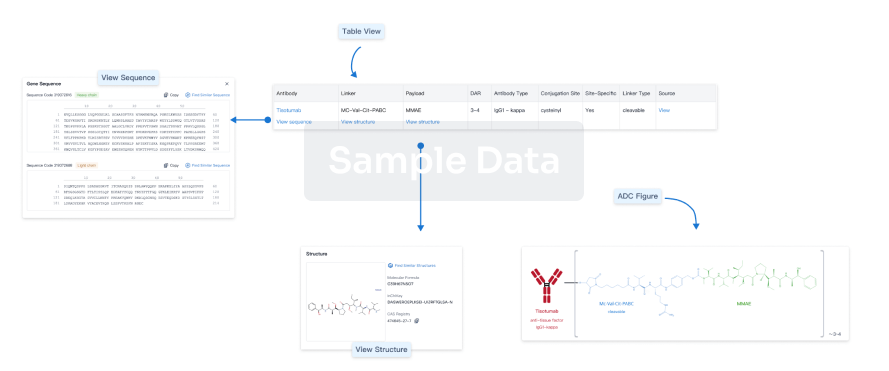
Structure/Sequence
Boost your research with our ADC technology data.
login
or
Related
14
Clinical Trials associated with TQB-2102NCT07043725
A Randomized, Open-label, Multicenter, Parallel-controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of TQB2102 for Injection Versus TCbHP in Neoadjuvant Treatment of Breast Cancer With Positive HER2 Expression
This is a randomized, open, positive drug control, multi center phase III study. Through the evaluation of tpCR, bpCR, ORR, EFS, IDFS, OS , AEs and other indicators, it proves the effectiveness and safety of TQB2102 for injection versus TCbHP in the neoadjuvant treatment of HER2 positive breast cancer patients.
Start Date01 Aug 2025 |
Sponsor / Collaborator |
NCT07003074
A Randomized, Open, Multicenter, Parallel-controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of TQB2102 for Injection Versus Docetaxel Plus Trastuzumab and Pertuzumab in the Treatment of Human Epidermal Growth Factor Receptor 2 (HER2) Positive Recurrent or Metastatic Breast Cancer
This Phase III trial adopts a randomized, open label, positive drug control, and multicenter trial design. Subjects who meet the criteria are randomly divided into 1:1 groups and receive treatment with TQB2102 injection or docetaxel combined with trastuzumab and pertuzumab, respectively.
Start Date23 Jun 2025 |
Sponsor / Collaborator |
NCT07008976
A Randomized, Open-label, Parallel-controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of TQB2102 for Injection Versus Trastuzumab Emtansine for Injection in Patients With HER2-positive Advanced Breast Cancer
This study adopted a randomized, open-label, positive drug-controlled, multi-center trial design. The primary endpoint was PFS evaluated by the Independent Review Committee (IRC). Eligible subjects were randomly assigned in a 1:1 ratio to receive either TQB2102 for injection or trastuzumab emtansine for injection.
Start Date01 Jun 2025 |
Sponsor / Collaborator |
100 Clinical Results associated with TQB-2102
Login to view more data
100 Translational Medicine associated with TQB-2102
Login to view more data
100 Patents (Medical) associated with TQB-2102
Login to view more data
12
Literatures (Medical) associated with TQB-210214 Nov 2024·JOURNAL OF MEDICINAL CHEMISTRY
Evaluation of Double Self-Immolative Linker-Based Antibody–Drug Conjugate FDA022-BB05 with Enhanced Therapeutic Potential
Article
Author: Yin, Sicheng ; Liu, Shuangxi ; Zhang, Yifan ; Yang, Tong ; Zhang, Wenbo ; Shen, Keyu ; Zhu, Shulei ; Xie, Qian ; Gao, Bei ; Wu, Yinghan ; Song, Ruiwen ; Cao, Xuemei ; Qiu, Xuefei ; Cheng, Zhiyang ; Wang, Baoxia ; Zhao, Teng ; Xu, Jun ; Wu, Jingsong ; Li, Weinan ; Guo, Qingsong ; Wang, Lei ; Lu, Wei
Typical antibody-drug conjugates (ADCs) with valine-alanine linkage, often conjugated with the amino group in payloads, face challenges when interacting with hydroxyl group-containing payloads. Herein, we introduced a transformative Val-Ala-based double self-immolative linker-payload platform, reshaping ADCs by optimizing hydroxyl group-containing payload integration. Utilizing this platform, FDA022-BB05 was successfully conjugated with the hydroxyl group-containing payload DXd using trastuzumab (FDA022) as the monoclonal antibody (mAb). FDA022-BB05 demonstrated enhanced stability, effective cathepsin B sensitivity, reduced cell proliferation, increased bystander killing, and targeted delivery. Notably, acute toxicity evaluations in diverse preclinical models indicated favorable safety profiles and tolerability, with a broad therapeutic index in HER2-positive and -negative xenografts. Overall, these compelling findings support the promising therapeutic potential of FDA022-BB05, emphasizing the significance of diverse linker-payload platform strategies. This ADC is a valuable addition to targeted cancer therapy development, currently advancing through phase I clinical trials.
01 Oct 2024·MOLECULAR CANCER THERAPEUTICS
FZ-AD005, a Novel DLL3-Targeted Antibody–Drug Conjugate with Topoisomerase I Inhibitor, Shows Potent Antitumor Activity in Preclinical Models
Article
Author: Wu, Jinsong ; Lu, Wei ; Shen, Jiafei ; Zhao, Teng ; Gao, Bei ; Yang, Tong ; Song, Ruiwen ; Xie, Qian ; Guo, Qingsong ; Lou, Sensen ; Li, Weinan ; Zhang, Yifan ; Zhu, Shulei ; Wang, Lei
Abstract:
Delta-like ligand 3 (DLL3) is overexpressed in small cell lung cancer (SCLC) and has been considered an attractive target for SCLC therapy. Rovalpituzumab tesirine was the first DLL3-targeted antibody–drug conjugate (ADC) to enter clinical studies. However, serious adverse events limited progress in the treatment of SCLC with rovalpituzumab tesirine. In this study, we developed a novel DLL3-targeted ADC, FZ-AD005, by using DXd with potent cytotoxicity and a relatively better safety profile to maximize the therapeutic index. FZ-AD005 was generated by a novel anti-DLL3 antibody, FZ-A038, and a valine–alanine (Val–Ala) dipeptide linker to conjugate DXd. Moreover, Fc-silencing technology was introduced in FZ-AD005 to avoid off-target toxicity mediated by FcγRs and showed negligible Fc-mediated effector functions in vitro. In preclinical evaluation, FZ-AD005 exhibited DLL3-specific binding and demonstrated efficient internalization, bystander killing, and excellent in vivo antitumor activities in cell line–derived xenograft and patient-derived xenograft models. FZ-AD005 was stable in circulation with acceptable pharmacokinetic profiles in cynomolgus monkeys. FZ-AD005 was well tolerated in rats and monkeys. The safety profile of FZ-AD005 was favorable, and the highest nonseverely toxic dose was 30 mg/kg in cynomolgus monkeys. In conclusion, FZ-AD005 has the potential to be a superior DLL3-targeted ADC with a wide therapeutic window and is expected to provide clinical benefits for the treatment of patients with SCLC.
01 Oct 2024·BIOORGANIC CHEMISTRY
Novel antibody-drug conjugates based on DXd-ADC technology
Review
Author: Chen, Yuchen ; Ye, Qiang ; Hu, Xuefang ; Bai, Lan ; Ren, Zhiwen ; Shi, Jianyou ; Hu, Yuan ; Chen, Rong
In recent years, antibody-drug conjugate (ADC) technology, which uses monoclonal antibodies (mAbs) to specifically deliver effective cytotoxic payloads to tumor cells, has become a promising method of tumor targeted therapy. ADCs are a powerful class of biopharmaceuticals that link antibodies targeting specific antigens and small molecule drugs with potent cytotoxicity via a linker, thus enabling selective destruction of cancer cells while minimizing systemic toxicity. DXd is a topoisomerase I inhibitor that induces DNA damage leading to cell cycle arrest, making it an option for ADC payloads. The DXd-ADC technology, developed by Daiichi Sankyo, is a cutting-edge platform that produces a new generation of ADCs with improved therapeutic metrics and has shown significant therapeutic potential in various types of cancer. This review provides a comprehensive assessment of drugs developed with DXd-ADC technology, with a focus on mechanisms of action, pharmacokinetics studies, preclinical data, and clinical outcomes for DS-8201a, U3-1402, DS-1062a, DS-7300a, DS-6157a, and DS-6000a. By integrating existing data, we aim to provide valuable insights into the current therapeutic status and future prospects of these novel agents.
1
News (Medical) associated with TQB-210212 Jun 2025
The year 2024 marked an unprecedented boom in the field of antibody-drug conjugates (ADCs). With multiple innovative drugs advancing smoothly from Phase III clinical trials to regulatory review and market approval, the outlook for cancer treatment has become increasingly promising. These breakthrough ADCs have not only demonstrated remarkable efficacy and safety profiles but have also provided more personalized and precise therapeutic options for patients with various types of cancer. Whether targeting Trop-2, HER2, or c-Met, these drugs have shown strong potential within their respective indications.
Throughout the year, several high-profile ADCs made pivotal progress in Phase III trials and quickly entered the new drug approval pipeline. Notable examples include Datopotamab Deruxtecan, Sacituzumab Tirumotecan, and Trastuzumab Rezetecan, all of which captured global attention through their unique mechanisms of action and compelling clinical data. Meanwhile, Telisotuzumab Vedotin and Becotatug Vedotin have emerged in their respective therapeutic areas by efficiently targeting specific antigens. These successes not only represent significant scientific advancement but also a transformative shift in cancer treatment paradigms.
ADCs in Phase III, Regulatory Review, or Market Launch in 20241. Trop-2–Targeted TherapiesDatopotamab Deruxtecan
Datopotamab Deruxtecan (also known as Dato-DXd or DS-1062) is a Trop-2–targeting antibody-drug conjugate co-developed by Daiichi Sankyo and AstraZeneca. Trop-2 (Trophoblast Cell Surface Antigen 2) is a protein highly expressed in various cancers. Datopotamab Deruxtecan combines a humanized anti–Trop-2 monoclonal antibody with a topoisomerase I inhibitor (DXd) payload, allowing it to selectively target and kill cancer cells.
The drug has been approved in Japan for the treatment of HR-positive/HER2-negative unresectable or recurrent breast cancer and has received Breakthrough Therapy Designation in the U.S. for treating EGFR-mutant non-small cell lung cancer (NSCLC). It is also under investigation for a range of solid tumors including NSCLC and triple-negative breast cancer (TNBC). Clinical trials have shown that Datopotamab Deruxtecan significantly reduces the risk of disease progression or death compared to traditional chemotherapy. However, common adverse events like anemia and thrombocytopenia, as well as the risk of interstitial lung disease (ILD), require careful monitoring.
Major downstream events of Trop-21Trop-2 is a transmembrane glycoprotein encoded by the TACSTD2 gene and is part of the tumor-associated calcium signal transducer (TACSTD) family. While normally restricted to epithelial tissues such as the placenta and kidney, Trop-2 is abnormally overexpressed in numerous malignancies.
Functionally, Trop-2 activates the ERK/MAPK signaling pathway, which plays a key role in cell proliferation, differentiation, survival, and apoptosis. This activation leads to increased expression of cyclins such as Cyclin D1 and Cyclin E, promoting cell cycle progression and tumor cell proliferation. In addition, Trop-2 regulates intracellular calcium signaling—critical for functions like muscle contraction, neurotransmitter release, and gene expression. It modulates calcium signaling by enriching RACK1 at the cell membrane, thereby reducing fibronectin–integrin β1 binding, which decreases cellular adhesion. This mechanism facilitates tumor cell detachment and metastatic spread.
TROP-2 mediates several intracellular signaling pathways2Datopotamab Deruxtecan utilizes a humanized anti–Trop-2 monoclonal antibody that specifically binds to Trop-2, which is highly expressed in many solid tumors. It is conjugated via a cleavable tetrapeptide linker to the potent topoisomerase I inhibitor DXd. Once internalized by Trop-2–expressing cancer cells, the linker is enzymatically cleaved, releasing DXd inside the cell. The released payload causes DNA damage and triggers apoptosis. Importantly, due to its membrane permeability, DXd can diffuse into neighboring tumor cells that express little or no Trop-2—a phenomenon known as the bystander effect.
Datopotamab Deruxtecan has demonstrated promising activity in cancers such as NSCLC and TNBC. For instance, in the TROPION-Lung02 trial, the drug combined with pembrolizumab ± platinum-based chemotherapy achieved favorable overall response rates (ORR) and disease control rates (DCR). In the BEGONIA trial, its combination with durvalumab led to an ORR of 79%, showing sustained tumor responses.
These results indicate that Datopotamab Deruxtecan offers an effective treatment option for patients with Trop-2–overexpressing cancers. However, it does carry risks of adverse events such as stomatitis, nausea, and appetite loss. Cases of serious ILD have also been reported, necessitating close monitoring during therapy to promptly manage any potential complications.
Sacituzumab Tirumotecan
Sacituzumab Tirumotecan, developed by Sichuan Kelun Botai Biomedicine, is another antibody-drug conjugate (ADC) targeting TROP-2. On November 22, 2024, it was approved for the first time in China for the treatment of adult patients with unresectable locally advanced or metastatic triple-negative breast cancer (TNBC) who have previously received at least two systemic therapies.
Sacituzumab Tirumotecan has demonstrated significant efficacy across multiple clinical studies. In a Phase II clinical trial involving patients with recurrent or metastatic cervical cancer, the combination of Sacituzumab Tirumotecan with pembrolizumab achieved an objective response rate (ORR) of 57.9%, underscoring its potential in treating this difficult-to-treat malignancy. Notably, in the subgroup of patients previously treated with anti–PD-1 therapy, the ORR was even higher at 68.8%. Similarly, in a Phase II study targeting gastric cancer and gastroesophageal junction adenocarcinoma, Sacituzumab Tirumotecan monotherapy showed promising efficacy, with an ORR of 22.0% and a disease control rate (DCR) of 80.5%, even among patients heavily pretreated with multiple lines of therapy.
In the pivotal Phase III trial OptiTROP-Breast01 for TNBC, Sacituzumab Tirumotecan demonstrated a median progression-free survival (mPFS) of 6.7 months compared to 2.5 months with standard chemotherapy, along with an improved ORR of 45.4%. These results mark a significant breakthrough in the treatment of advanced TNBC.
The development journey of Sacituzumab Tirumotecan has been marked by several milestones. Leveraging its advanced OptiDC™ platform, Kelun Botai successfully developed this TROP-2–targeting ADC and secured approval from the National Medical Products Administration (NMPA) on November 22, 2024, making it the first domestically approved TROP-2–targeting ADC in China. It is specifically indicated for adults with unresectable locally advanced or metastatic TNBC who have received at least two prior systemic therapies.
Beyond its approved use in TNBC, Sacituzumab Tirumotecan also shows potential across a range of other cancers, including EGFR-mutant non-small cell lung cancer (NSCLC), platinum-sensitive ovarian cancer, and squamous cell carcinoma of the cervix. In EGFR-mutant NSCLC studies, the drug has shown a high ORR and extended PFS, suggesting it may reshape the treatment landscape for this subtype.
Several other Trop-2–targeting ADCs currently in Phase III clinical trials are also worth noting:
·ESG-401, jointly developed by Shanghai Shijian Biotechnology and Levena Suzhou Biopharma, employs a novel, highly stable, biodegradable linker to conjugate a humanized anti–Trop-2 monoclonal antibody with the topoisomerase I inhibitor SN-38. It is being investigated for multiple solid tumors.
·FDA018, developed by Shanghai Fudan Zhangjiang Bio Pharmaceutical, is an ADC that combines an anti–Trop-2 monoclonal antibody with SN-38, intended for the treatment of various cancers, including TNBC.
·Tizetatug Rezetecan, though not primarily targeting Trop-2, is an ADC developed by Suzhou Suncadia Biopharmaceuticals that targets other tumor-associated antigens and is under development for alternative indications.
2. c-Met Targeting DrugsTelisotuzumab Vedotin
Telisotuzumab vedotin (brand name Teliso-V), developed by AbbVie, Inc., is an antibody-drug conjugate (ADC) that targets the c-Met protein—a receptor tyrosine kinase that is overexpressed in a variety of tumors. This drug combines a microtubule inhibitor (MMAE) with a humanized monoclonal antibody against c-Met, enabling precise targeting and killing of cancer cells. Telisotuzumab vedotin is primarily intended for the treatment of c-Met–positive non-small cell lung cancer (NSCLC), especially in EGFR wild-type, non-squamous patients who have already undergone prior therapies. Although it is not yet approved for commercial use, AbbVie submitted a Biologics License Application (BLA) to the U.S. FDA on September 27, 2024, seeking accelerated approval for use in specific subtypes of advanced NSCLC. The drug is administered via intravenous infusion, with dosage and treatment cycles determined according to clinical guidelines. If approved, Teliso-V is expected to become the first-in-class therapy specifically designed for patients with c-Met overexpressing NSCLC.
Different downstream signaling pathways activated through c-MET and its interactive other membrane receptors3c-Met, also known as hepatocyte growth factor receptor (HGFR), is a protein encoded by the proto-oncogene c-MET. It plays a pivotal role in various physiological processes, including embryonic development, wound healing, and tissue regeneration. Activation of c-Met occurs upon binding to its only known ligand, hepatocyte growth factor (HGF). Once activated, c-Met triggers a cascade of downstream signaling pathways such as RAS-RAF-MEK-ERK, PI3K-AKT, and STAT, which together regulate cell proliferation, survival, migration, differentiation, and morphogenesis.
Structurally, c-Met is a heterodimer consisting of an α-chain and a β-chain, both derived from the cleavage of a single precursor polypeptide, connected by disulfide bonds. Its extracellular domain contains three major regions—Sema, PSI, and IPT domains—that are responsible for HGF binding. HGF binding induces receptor dimerization and autophosphorylation, thereby initiating downstream signal transduction.
Under normal conditions, c-Met activity is tightly regulated. However, in many cancers, c-Met is abnormally activated or overexpressed, contributing to uncontrolled tumor growth, invasion, and metastasis. Aberrant c-Met signaling has been documented in multiple cancer types, including NSCLC, gastric cancer, and breast cancer. Additionally, c-Met dysregulation can lead to resistance against certain targeted therapies, such as EGFR tyrosine kinase inhibitors (TKIs).
Telisotuzumab vedotin comprises three key components:
·A humanized monoclonal antibody targeting c-Met
·A cytotoxic payload, monomethyl auristatin E (MMAE)
·A cleavable linker connecting the two
The antibody component specifically binds to c-Met proteins overexpressed on the surface of tumor cells. Upon binding, the entire ADC is internalized into the cell. Inside the cell, enzymatic or acidic conditions cleave the linker, releasing MMAE. MMAE disrupts the microtubule network, preventing cell division and leading to apoptosis. Because the cytotoxin is only released inside the target cell, the drug minimizes damage to surrounding healthy tissue, improving both safety and target specificity.
In clinical studies, Telisotuzumab vedotin has shown notable efficacy, particularly among NSCLC patients with high c-Met expression. For example, in the LUMINOSITY Phase II trial, the objective response rate (ORR) was 53.8% in EGFR wild-type, non-squamous NSCLC patients with high c-Met expression, compared to 25% in those with moderate expression. The drug also demonstrated favorable tolerability, with common side effects including peripheral sensory neuropathy and fatigue. These results highlight the drug’s potential as a valuable treatment option, particularly for patients with limited response to conventional therapies.
In addition to Telisotuzumab vedotin, Telisotuzumab adizutecan (also known as ABBV-400) is another c-Met–targeting ADC developed by AbbVie. It employs a specific linker to couple a monoclonal antibody with a cytotoxic payload, designed to selectively eliminate tumor cells expressing c-Met. The antibody binds to the c-Met receptor on cancer cell surfaces, is internalized, and subsequently releases the cytotoxic compound, inducing cell death. Its primary indications include non-small cell lung cancer and colorectal cancer.
3. HER2-Targeted TherapyTrastuzumab Rezetecan
Trastuzumab Rezetecan (development code SHR-A1811), developed independently by Hengrui Pharmaceutical Group, is an antibody-drug conjugate (ADC) targeting human epidermal growth factor receptor 2 (HER2). This novel agent offers a new treatment option for a range of advanced solid tumors with HER2 expression or mutation, including—but not limited to—HER2-mutant non-small cell lung cancer (NSCLC), ovarian cancer, recurrent platinum-resistant primary peritoneal cancer, colorectal cancer, as well as HER2-positive gastric and breast cancers. Trastuzumab Rezetecan binds to HER2-positive tumor cells and is internalized; once inside the lysosome, it releases its cytotoxic payload, inducing cell cycle arrest and ultimately leading to apoptosis.
HER2, also known as c-erbB-2 or ERBB2, is a membrane-bound receptor protein and a member of the human epidermal growth factor receptor (EGFR) family. Encoded by the HER2 gene located on chromosome 17q21, HER2 is a transmembrane glycoprotein with tyrosine kinase activity. It activates downstream signaling pathways critical for cell growth, differentiation, proliferation, and survival, often through heterodimerization with other family members such as EGFR/HER1, HER3, and HER4.
Mechanism of action of agents targeting HER24HER2 is overexpressed or amplified in approximately 20–30% of primary invasive breast cancers, a phenomenon typically associated with poor prognosis. HER2-positive breast cancer is characterized by rapid cell proliferation, high aggressiveness, and resistance to certain conventional chemotherapy regimens. However, the emergence of HER2-targeted biologics such as trastuzumab, pertuzumab, and lapatinib has significantly improved survival outcomes in HER2-positive patients.
Accurate HER2 status assessment is critical for therapeutic decision-making in breast cancer. Common diagnostic tools include immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH). IHC assesses HER2 protein expression, while FISH detects gene amplification. HER2 positivity is defined as IHC 3+ or FISH-positive; IHC 2+ requires further confirmation with in situ hybridization (ISH), while IHC 0 or 1+ is considered HER2-negative.
Trastuzumab Rezetecan comprises three components:
·A humanized monoclonal antibody targeting HER2
·A novel topoisomerase I inhibitor payload (SHR9265)
·A cleavable tetrapeptide linker
In vivo, the ADC specifically binds HER2 on tumor cells and is internalized. Once inside lysosomes, the linker is enzymatically cleaved, releasing the cytotoxic payload. This payload induces cell cycle arrest and apoptosis in cancer cells.
Trastuzumab Rezetecan has been granted Breakthrough Therapy Designation by multiple National Medical Products Administration (NMPA) Centers for Drug Evaluation (CDE) in China for several indications, including:
·HER2-expressing platinum-resistant recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer
·HER2-low recurrent or metastatic breast cancer
·HER2-positive recurrent or metastatic breast cancer
The drug has also been proposed for priority review, particularly for the treatment of locally advanced or metastatic HER2-mutant NSCLC in adults who have previously received at least one line of systemic therapy.
In a Phase I/II clinical trial targeting HER2-mutant NSCLC, Trastuzumab Rezetecan demonstrated promising clinical activity, durable responses, and a favorable safety profile. Among patients in the full-dose cohort, the median progression-free survival (mPFS) was 9.5 months, with an objective response rate (ORR) of 38.1% and a disease control rate (DCR) exceeding 90%. The median duration of response (DoR) was 10.3 months. As a next-generation ADC, Trastuzumab Rezetecan not only broadens treatment options for HER2-positive cancers but also provides renewed hope for patients who have developed resistance to conventional chemotherapy.
Other HER2-Targeted Antibody-Drug Conjugates in Phase III Clinical Development
In addition to the already approved Trastuzumab Rezetecan, several HER2-targeted antibody-drug conjugates (ADCs) are currently undergoing Phase III clinical trials, including TQB2102, GQ-1005, and DB-1303.
TQB2102, developed by Nanjing Shunxin Pharmaceutical, is designed to bind specifically to HER2-positive tumor cell surfaces. Once bound, it is internalized into the cancer cell and trafficked to the lysosome, where it releases a cytotoxic payload. This release induces DNA damage and cell death. In addition to its direct cytotoxic effect, TQB2102 is engineered to inhibit the HER2 signaling pathway and may exert a bystander effect, enabling it to kill adjacent tumor cells that may not strongly express HER2—addressing tumor heterogeneity.
DB-1303, developed by Duality Biologics, is composed of a trastuzumab biosimilar antibody backbone targeting HER2, a stable, enzyme-cleavable peptide linker, and a proprietary topoisomerase I inhibitor payload. This structure grants DB-1303 potent anti-tumor activity in both HER2-positive and HER2-low expressing tumors, along with a broad therapeutic window. The bystander effect of DB-1303 allows it to not only eliminate directly targeted HER2-positive tumor cells but also affect neighboring non-targeted cancer cells—an important feature for addressing intratumoral heterogeneity.
GQ-1005, developed by GeneQuantum Healthcare (Suzhou) Co., Ltd., is another HER2-targeted ADC that combines a monoclonal antibody specific to HER2 with a cytotoxic payload. The payload is a topoisomerase I inhibitor (ED-04), which is conjugated to the antibody through the proprietary Ex-0115 linker-payload technology platform. Each GQ-1005 molecule carries approximately 7 to 8 toxin units, which are released upon internalization into tumor cells, leading to cellular apoptosis. This design enables precise targeting and potent cytotoxicity against HER2-expressing cancer cells.
4. EGFR-Targeted Antibody-Drug ConjugatesBecotatug Vedotin
Becotatug vedotin, formerly known as MRG003, is an antibody-drug conjugate (ADC) developed by Shanghai Miracogen, targeting the epidermal growth factor receptor (EGFR). The drug is composed of a humanized IgG1κ monoclonal antibody linked to the microtubule-disrupting agent monomethyl auristatin E (MMAE) via a cleavable linker. Becotatug vedotin is designed to treat multiple EGFR-positive tumors, including but not limited to nasopharyngeal carcinoma (NPC), metastatic head and neck squamous cell carcinoma, gastroesophageal junction adenocarcinoma, and advanced non-small cell lung cancer (NSCLC).
Becotatug vedotin has received Breakthrough Therapy Designation from the National Medical Products Administration (NMPA) of China for the treatment of recurrent or metastatic NPC, and its New Drug Application (NDA) has been accepted by the NMPA. In the United States, it has also been granted Breakthrough Therapy, Orphan Drug, and Fast Track designations by the FDA for the same indication. However, the commercial name, exact launch timeline, and dosing regimen have not yet been disclosed. As with most ADCs, intravenous infusion is the likely route of administration. Jin Mantle is the lead organization advancing the drug into global Phase III clinical trials, in collaboration with Lepu Biopharma.
EGFR could trigger downstream PI3K/Akt and MAPK signaling5EGFR, a member of the ErbB receptor family (which also includes HER2/neu (ErbB2), HER3 (ErbB3), and HER4 (ErbB4)), consists of three main domains: an extracellular ligand-binding domain, a single transmembrane helix, and an intracellular tyrosine kinase domain. Upon ligand binding (e.g., EGF or TGF-α), EGFR dimerizes, triggering autophosphorylation of its tyrosine residues and initiating downstream signaling cascades.
EGFR plays a vital role in various biological processes such as embryonic development, tissue repair, and epithelial homeostasis. It also contributes to immune regulation, inflammatory responses, and neurological functions. However, EGFR overexpression or mutation can drive uncontrolled cell proliferation and tumor development, as observed in cancers such as NSCLC, colorectal cancer, and head and neck squamous cell carcinoma.
With advances in understanding the EGFR signaling pathway, numerous EGFR-targeted therapies have been developed to inhibit signal transduction and suppress tumor growth. While first- and second-generation EGFR inhibitors have shown clinical success, drug resistance remains a challenge, prompting the development of third-generation inhibitors and novel strategies, including imaging-guided personalized therapies using radiolabeled probes.
Becotatug vedotin combines an EGFR-targeting monoclonal antibody with a potent cytotoxin (MMAE) via a degradable linker. This design enables precise targeting of EGFR-overexpressing cancer cells. Upon binding, the drug is internalized, and the linker is cleaved within the lysosome, releasing MMAE, which disrupts microtubules and induces cancer cell death.
The drug was primarily developed for patients with recurrent or metastatic NPC, especially those unresponsive to traditional chemotherapy or PD-1/PD-L1 inhibitors. In a Phase IIa clinical trial, Becotatug vedotin demonstrated promising efficacy, with objective response rates (ORR) ranging from 39.3% to 55.2% across various dosing cohorts. These results suggest strong potential as a therapeutic option. The drug has been submitted for marketing approval in China and granted priority review, indicating that it may soon be available for clinical use.
Another EGFR-targeted ADC, SYS-6010 (also known as CPO-301), developed by CSPC Megalith Biopharmaceutical, is engineered to specifically bind EGFR-mutated tumor cells and release its cytotoxic payload upon internalization. Preclinical studies have shown robust anti-tumor activity, especially against NSCLC models harboring triple EGFR mutations (Exon19Del, T790M, and C797S).
SummaryThe development of ADCs in 2024 has led to major milestones across several key targets and representative agents:
·Trop-2-targeted ADCs: Datopotamab Deruxtecan (by Daiichi Sankyo and AstraZeneca) and Sacituzumab tirumotecan (by Kelun Botai Biomedicine) demonstrated impressive efficacy and safety, especially in TNBC and NSCLC.
·HER2-targeted ADCs: Trastuzumab Rezetecan (by Hengrui Pharmaceutical) offered new hope for patients with HER2-expressing or mutant advanced solid tumors.
·c-Met-targeted ADCs: Telisotuzumab vedotin (by AbbVie) was developed specifically for c-Met-positive NSCLC, representing a targeted, first-in-class approach.
·EGFR-targeted ADCs: Becotatug vedotin provided a promising treatment option for recurrent/metastatic NPC and other EGFR-positive cancers.
These innovations are more than technical achievements—they reflect the advancement of precision medicine. By selectively targeting tumor-specific antigens and minimizing damage to healthy tissue, ADCs significantly enhance treatment efficacy and improve patient quality of life.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Refrence
1.Qiu S, Zhang J, Wang Z, Lan H, Hou J, Zhang N, Wang X, Lu H. Targeting Trop-2 in cancer: Recent research progress and clinical application. Biochim Biophys Acta Rev Cancer. 2023 Jul;1878(4):188902. doi: 10.1016/j.bbcan.2023.188902. Epub 2023 Apr 29. PMID: 37121444.2.Parisi C, Mahjoubi L, Gazzah A, Barlesi F. TROP-2 directed antibody-drug conjugates (ADCs): The revolution of smart drug delivery in advanced non-small cell lung cancer (NSCLC). Cancer Treat Rev. 2023 Jul;118:102572. doi: 10.1016/j.ctrv.2023.102572. Epub 2023 May 19. PMID: 37230055.3.Raj S, Kesari KK, Kumar A, Rathi B, Sharma A, Gupta PK, Jha SK, Jha NK, Slama P, Roychoudhury S, Kumar D. Molecular mechanism(s) of regulation(s) of c-MET/HGF signaling in head and neck cancer. Mol Cancer. 2022 Jan 26;21(1):31. doi: 10.1186/s12943-022-01503-1. PMID: 35081970; PMCID: PMC8790852.4.Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. 2020 Jan;17(1):33-48. doi: 10.1038/s41571-019-0268-3. Epub 2019 Sep 23. PMID: 31548601.5.Liu Q, Yu S, Zhao W, Qin S, Chu Q, Wu K. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol Cancer. 2018 Feb 19;17(1):53. doi: 10.1186/s12943-018-0793-1. PMID: 29455669; PMCID: PMC5817859.
100 Deals associated with TQB-2102
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Advanced HER2-Positive Breast Carcinoma | Phase 3 | China | 01 Jun 2025 | |
| Metastatic human epidermal growth factor 2 positive carcinoma of breast | Phase 3 | China | 01 Jun 2025 | |
| HR-positive/HER2-low Breast Carcinoma | Phase 3 | China | 24 Sep 2024 | |
| HER2-Low Breast Carcinoma | Phase 3 | China | 01 Aug 2024 | |
| Female Genital Neoplasms | Phase 2 | China | 13 Mar 2025 | |
| HER2 Positive Gastroesophageal Adenocarcinoma | Phase 2 | China | 21 Nov 2024 | |
| GRPR Positive/ER Positive/HER2 Negative Breast Cancer | Phase 2 | China | 14 Nov 2024 | |
| Human epidermal growth factor 2 negative carcinoma of breast | Phase 2 | China | 14 Nov 2024 | |
| HER2-negative breast cancer | Phase 2 | China | 05 Nov 2024 | |
| Locally Advanced Lung Non-Small Cell Carcinoma | Phase 2 | China | 06 Aug 2024 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 2 | 52 | xuovxajjzx(hypozoxenf) = dgfnoeqnwz gblaliredz (szsbsjywzw, 36.9% - 76.7) View more | Positive | 30 May 2025 | |||
xuovxajjzx(hypozoxenf) = booanzdoip gblaliredz (szsbsjywzw, 56.3% - 91) View more | |||||||
Phase 1 | HER2-Low Breast Carcinoma HER2 low-expressing | 73 | tlzpqaekpu(utxfkuieko) = agzqjcndza flyjdifiad (aptssmtzkv ) | Positive | 30 May 2025 | ||
tlzpqaekpu(utxfkuieko) = ahczetoaqs flyjdifiad (aptssmtzkv ) | |||||||
Phase 1 | Advanced Malignant Solid Neoplasm HER2 positive | HER2 low (HER2 1+ or HER2 2+ and FISH negative) | 181 | svelvnlzoo(zzsclabeag) = Only one patient had grade 2 interstitial lung disease (ILD) until the cutoff date rqkmogowyk (mqqcllzfpm ) | Positive | 30 May 2025 | ||
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
Deal
Boost your decision using our deal data.
login
or
Core Patent
Boost your research with our Core Patent data.
login
or
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or
Biosimilar
Competitive landscape of biosimilars in different countries/locations. Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3.
login
or
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free

