Request Demo
Last update 13 Dec 2025
Fesomersen
Last update 13 Dec 2025
Overview
Basic Info
Drug Type ASO |
Synonyms fesomersen, Fesomersen Sodium, IONIS-FXI-L Rx + [3] |
Target |
Action inhibitors |
Mechanism F11 inhibitors(Coagulation factor XI inhibitors) |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
License Organization |
Drug Highest PhasePendingPhase 2 |
First Approval Date- |
Regulation- |
Login to view timeline
Structure/Sequence
Boost your research with our RNA technology data.
login
or
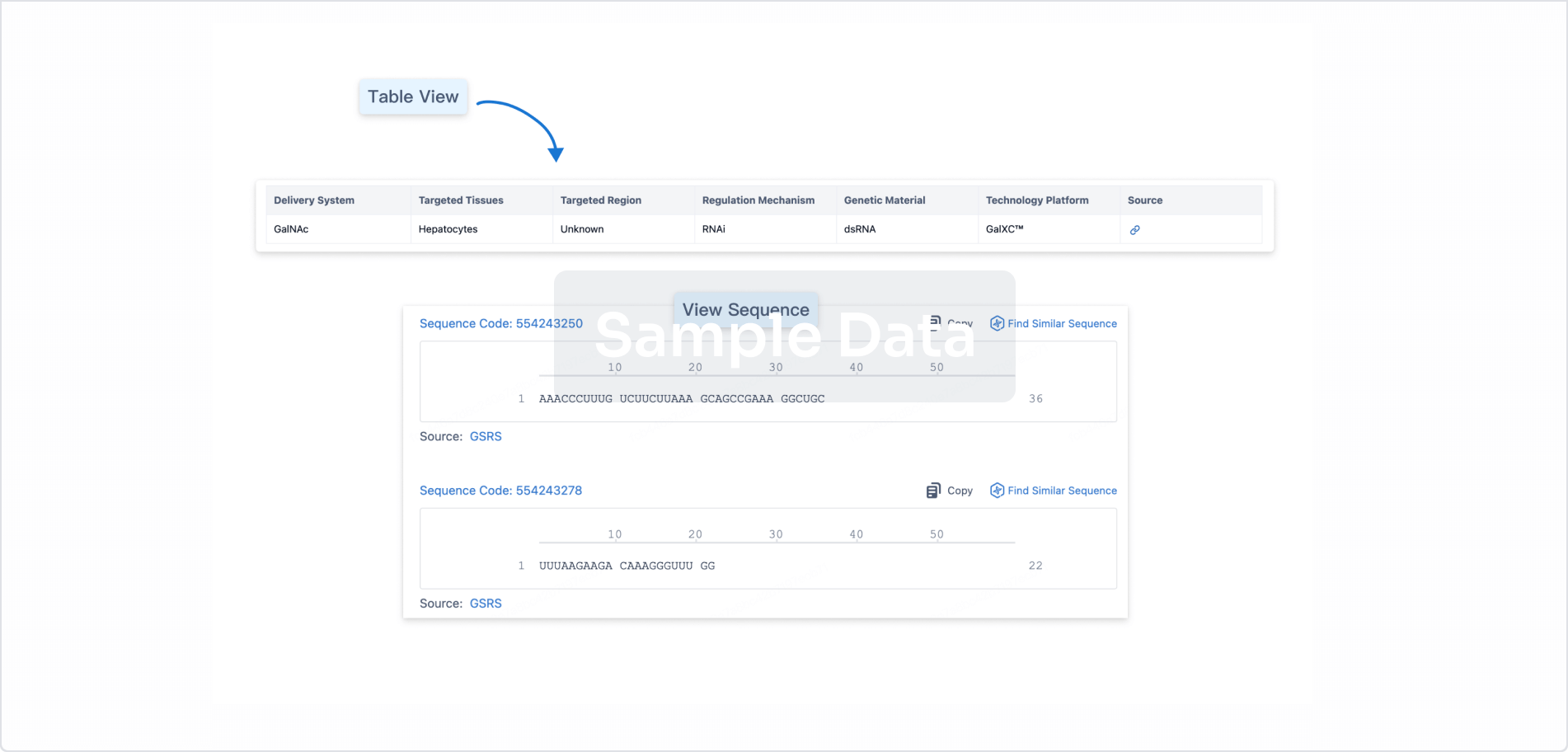
Sequence Code 525736303

Source: *****
Related
2
Clinical Trials associated with FesomersenCTR20211265
一项在中国健康男性受试者中评价BAY 2976217单次皮下给药的安全性、耐受性、药代动力学和药效学特征的单盲、安慰剂对照、随机、剂量递增I期研究
[Translation] A single-blind, placebo-controlled, randomized, dose-escalation Phase I study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of a single subcutaneous dose of BAY 2976217 in healthy Chinese male subjects
在健康受试者中评价BAY 2976217单次皮下注射(SC)给药的安全性、耐受性、药代动力学和药效学
[Translation]
To evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of a single subcutaneous (SC) injection of BAY 2976217 in healthy subjects
Start Date17 Jul 2021 |
Sponsor / Collaborator  Bayer AG Bayer AG [+2] |
NCT04534114
Factor XI LICA to Reduce Thrombotic Events in End-Stage Renal Disease Patients on Hemodialysis: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study of the Safety, Pharmacokinetics, and Pharmacodynamics of Multiple Doses of BAY 2976217
Patients whose kidneys are no longer able to work as they should and require treatment to filter wastes from the blood (hemodialysis) are at high risk for blood clots that form in blood vessels (thrombosis) blocking blood flow that causes heart attacks, strokes, and other life-threatening conditions. BAY2976217 is under clinical development for prevention of thrombosis. The goal of the study is to learn more about the safety of BAY2976217, how it is tolerated and the way the body absorbs, distributes and gets rid of the study dug given as multiple doses in participants with renal impairment who require hemodialysis.
Start Date04 Sep 2020 |
Sponsor / Collaborator |
100 Clinical Results associated with Fesomersen
Login to view more data
100 Translational Medicine associated with Fesomersen
Login to view more data
100 Patents (Medical) associated with Fesomersen
Login to view more data
7
Literatures (Medical) associated with Fesomersen01 Jul 2024·KIDNEY INTERNATIONAL
Factor XI inhibition in hemodialysis patients: the safer anticoagulation?
Article
Author: Floege, Jürgen ; Stamellou, Eleni ; Noels, Heidi
Chronic hemodialysis patients exhibit an excessive cardiovascular risk and a marked increase in both thromboembolism and bleeding episodes. Factor XI inhibition may provide anticoagulation, with a low risk of bleeding, and several factor XI inhibitors, including fesomersen, an antisense oligonucleotide, are under development. Recently, a phase 2 study of fesomersen showed a good safety profile in chronic hemodialysis patients and suggested that clotting rates of the arteriovenous fistula and the dialysis circuit are lower.
01 Jul 2024·Kidney international
A Phase II randomized controlled trial evaluated antithrombotic treatment with fesomersen in patients with kidney failure on hemodialysis
Article
Author: Pap, Ákos F ; Willmann, Stefan ; Lensing, Anthonie W A ; Eikelboom, John ; Mahaffey, Kenneth W ; Thadhani, Ravi I ; Solms, Alexander ; Ingham, Sheila J M ; Walsh, Michael ; Hodge, Sophie ; Winkelmayer, Wolfgang C ; Thelen, Kirstin
Patients with kidney failure on hemodialysis (KF-HD) are at high risk for both atherothrombotic events and bleeding. This Phase IIb study evaluated the dose-response of fesomersen, an inhibitor of hepatic Factor XI expression, versus placebo, for bleeding and atherothrombosis in patients with KF-HD. Patients were randomized to receive fesomersen 40, 80, or 120 mg once-monthly, or matching placebo, for up to 12 months. The primary safety endpoint was a composite of major bleeding and clinically relevant non-major bleeding (MB/CRNMB). Exploratory endpoints included post-dialysis arterio-venous (AV)-access bleeding, major atherothrombotic events (composite of fatal or non-fatal myocardial infarction, ischemic stroke, acute limb ischemia/major amputation, systemic embolism, symptomatic venous thromboembolism), AV-access thrombosis, and clotting of the hemodialysis circuit. Of 308 participants randomized, 307 received study treatment and were analyzed. Fesomersen led to a dose-dependent and sustained reduction of steady-state median FXI levels by 53.6% (40 mg group), 71.3% (80 mg group), 86.0% (120 mg group), versus 1.9% in the placebo group. MB/CRNMB events occurred in 6.5% (40 mg group), 5.1% (80 mg group), 3.9% (120 mg group), and in 4.0% of those receiving placebo (pooled fesomersen versus placebo P = 0.78). Major atherothrombotic events occurred in 1 patient (1.3%) in each treatment arm. MB/CRNMB bleeding and post-dialysis AV-access bleeding were not related to predicted FXI levels. Lower predicted FXI levels were associated with reductions in hemodialysis circuit clotting (P = 0.002) and AV-access thrombosis (P = 0.014). In patients with KF-HD, fesomersen produced a dose-dependent reduction in FXI levels associated with similar rates of major bleeding compared with placebo. REGISTRATION: URL: https://www.clinicaltrials.gov; unique identifier: NCT04534114.
01 Apr 2024·Clinical and translational science
Pharmacokinetics, pharmacodynamics, and safety of fesomersen, a novel antisense inhibitor of factor XI, in healthy Chinese, Japanese, and Caucasian volunteers
Article
Author: Willmann, Stefan ; Krieg, Eva ; Chen, Huijun ; Hashizume, Kensei ; Friedrichs, Frauke ; Yu, Rosie ; Mukaida, Yuki ; Liu, Tianxing ; Solms, Alexander ; Schwers, Stephan ; Thelen, Kirstin
Abstract:
The inhibition of coagulation factor XI (FXI) presents an attractive approach for anticoagulation as it is not expected to increase the risk of clinically relevant bleeding and is anticipated to be at least as effective as currently available anticoagulants. Fesomersen is a conjugated antisense oligonucleotide that selectively inhibits the expression of FXI. The article describes three clinical studies that investigated the safety, pharmacokinetic (PK), and pharmacodynamic (PD) profiles of fesomersen after subcutaneous (s.c.) injection to healthy participants. The studies included participants from diverse ethnic backgrounds (Caucasian, Japanese, and Chinese). Fesomersen demonstrated good safety and tolerability in all three studies. No major bleeding events were observed. After single‐dose s.c. injection, fesomersen was rapidly absorbed into the systemic circulation, with maximum fesomersen‐equivalent (fesomersen‐eq) concentrations (Cmax) in plasma observed within a few hours. After reaching Cmax, plasma fesomersen‐eq concentrations declined in a biphasic fashion. The PD analyses showed that the injection of fesomersen led to dose‐dependent reductions in FXI activity and increases in activated partial thromboplastin time (aPTT). The maximum observed PD effects were reached between Day 15 and 30, and FXI activity and aPTT returned to near‐baseline levels by Day 90 after a single dose. The PK/PD profiles after a single injection were similar among the various ethnic groups. Collectively, the study results suggest that fesomersen has a favorable safety profile and predictable and similar PK and PD profiles across Chinese, Japanese, and Caucasian participants.
13
News (Medical) associated with Fesomersen11 Jan 2023
Ionis CEO Brett Monia, Ph.D., is still hopeful about tofersen's approval chances as the clinical prospects of two other meds head in different directions.
Ionis Pharmaceuticals once had AstraZeneca involved with and spied a bright future for PCSK9 inhibitor ION449. Now, after the U.K. pharma backed out last year, CEO Brett Monia, Ph.D., thinks the drug may have missed its shot.
“PCSK9 is not a good match for us to bring forward through development,” Monia said in an interview with Fierce Biotech Wednesday morning ahead of the company’s presentation at the J.P. Morgan Healthcare Conference. “The investment in phase 3 is massive.”
Monia said the therapy will be hard to partner again due to the competition in the field and the strenuousness of planning a phase 3 trial. The reason for that, Monia explained, is that you likely need to conduct a cardiovascular outcomes trial to get payers to rally behind the drug. That’s time and money the company doesn’t want to put up. He added that a head-to-head comparison trial would also likely be needed to really win over the market.
AstraZeneca elected to walk away from ION449 in November after getting a look at phase 2b data in patients with hypercholesterolemia. If Ionis can’t find another partner, it’s likely to fall out of the pipeline.
“It really is a good-looking drug, it just probably came a little bit too late to the game,” Monia said. The drug would face rival therapies such as Sanofi and Regeneron's Praluent and Amgen's Repatha.
It’s a different story for fesomersen, which was returned to Ionis from Bayer after being tested in patients with end-stage renal disease. Monia says that there’s been greater interest in the factor XI drug compared to ION449, given the quality of the drug and market competitiveness.
Although Ionis has received inbound interest, Monia said locking up a new partnership this quarter would be too aggressive of a timeline given the med was just returned in the third quarter of 2022.
“Partnerships like these have a gestation period of usually about a year,” he said.
The diverging paths of ION449 and fesomersen come as Ionis is bracing for three new commercial products, furthering Monia’s goal of making Ionis a fully integrated biotech company rather than a licensing behemoth.
Included among the three is tofersen, a Biogen-partnered treatment for SOD1 amyotrophic lateral sclerosis. The FDA action date was pushed back in October 2022 from late January to late April, and an advisory committee meeting has not yet been scheduled, although Monia says he’s expecting and hoping for one.
The drug failed a phase 3 trial in 2021, but included in the approval application were 12-month data showing clinical improvements and better quality of life when tofersen was administered earlier.

Phase 3Phase 2
09 Nov 2022
Advanced key priorities: initiated manufacturing infrastructure project to support growth
Increased 2022 cash and investments guidance
CARLSBAD, Calif., Nov. 9, 2022 /PRNewswire/ -- Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) (the "Company") today reported financial results for the third quarter of 2022. Financial results are summarized below:
Financial Highlights
Revenue increased 20% for the third quarter of 2022 and 18% on a year-to-date basis compared to the same periods last year driven by significant partner payments earned across multiple programs
Non-GAAP operating expenses increased 5% for the third quarter of 2022 and 13% on a year-to-date basis compared to the same periods last year driven by advancing Phase 3 pipeline
Entered into a long-term lease in October 2022 to construct a new manufacturing facility supporting continued growth
Entered into a sale-leaseback transaction in October 2022 for several real estate assets, generating net proceeds of $240 million plus full funding to expand R&D campus
Reaffirmed 2022 P&L guidance; increased cash and investments guidance to approximately $2.0 billion
Late-Stage Pipeline Highlights
Presented positive data from the Phase 3 NEURO-TTRansform study of eplontersen in patients with polyneuropathy caused by hereditary TTR amyloidosis; on track to file U.S. New Drug Application this year
Expanded enrollment in the Phase 3 CARDIO-TTRansform study of eplontersen in patients with ATTR cardiomyopathy; data still expected first half of 2025
NDA for tofersen was accepted and granted priority review by the FDA; Prescription Drug User Fee Act date of April 25, 2023
Initiated CORE2, a confirmatory Phase 3 study of olezarsen in patients with severe hypertriglyceridemia (SHTG)
Initiated ESSENCE, a supporting Phase 3 study of olezarsen in patients with SHTG or hypertriglyceridemia and cardiovascular disease
Mid-Stage Pipeline Highlights
GSK presented positive end of study data from the Phase 2b B-Clear study of bepirovirsen demonstrating potential for functional cures in patients with chronic hepatitis B; GSK plans to advance bepirovirsen into Phase 3 development in the first half of 2023
Presented positive data from the Phase 2 study of IONIS-FB-LRx in patients with immunoglobulin A nephropathy; Roche plans to advance IONIS-FB-LRx into Phase 3 development in the first half of 2023
Bayer presented positive data from the Phase 2b study of fesomersen in patients with end-stage renal disease; Ionis regained rights to fesomersen from Bayer and is assessing next steps
Roche presented the Phase 2 GENERATION HD2 study design of tominersen in Huntington's disease patients; Roche plans to begin enrollment in early 2023
Reported ION449 (AZD8233) targeting PCSK9 met the primary endpoint in Phase 2b SOLANO study in patients with hypercholesterolemia; based on pre-specified efficacy criteria, AstraZeneca is not advancing ION449
"We have made significant progress on our key priorities this year, building our commercial pipeline, delivering an abundance of new medicines to the market and expanding and diversifying our technology. We delivered positive data from eight key programs, positioning us to potentially add two new marketed medicines to our portfolio and expand our rich Phase 3 pipeline to eight medicines next year. Additionally, we have recently begun work on a manufacturing facility to support our pipeline growth," said Brett P. Monia, Ph.D., chief executive officer of Ionis. "As we advance our near-term opportunities, including filing the NDA this year for eplontersen, and expanding our rich late-stage pipeline, we are well positioned to drive increasing value for all stakeholders."
Third Quarter 2022 Financial Results
Revenue
Ionis' revenue was comprised of the following:
Total revenue for the three and nine months ended September 30, 2022 increased 20 percent and 18 percent compared to the same periods last year, respectively. The increase was driven by significant payments Ionis earned across multiple partnered programs. R&D revenue for the nine months ended September 30, 2022 included $85 million from Biogen for advancing several neurology disease programs, $63 million from Roche for licensing and advancing IONIS-FB-LRx and $55 million from AstraZeneca for its share of the global Phase 3 development costs for eplontersen.
Commercial revenue for the three and nine months ended September 30, 2022 decreased 15 percent and 13 percent compared to the same periods last year, respectively. SPINRAZA royalties for the three and nine months ended September 30, 2022 decreased 7 percent and 12 percent compared to the same periods last year, respectively. In the U.S., SPINRAZA sales were flat in the first nine months of 2022 compared to the same period last year. Outside of the U.S. SPINRAZA royalties were lower due to lower SPINRAZA product sales primarily due to decreased pricing, foreign currency exchange and competition. TEGSEDI and WAYLIVRA revenue was also lower due to the shift to distribution fees in 2021.
Operating Expenses
Ionis' non-GAAP operating expenses increased for the three and nine months ended September 30, 2022 compared to the same periods in 2021, in line with expectations. For both periods, higher R&D expenses were driven by the expanded number of Phase 3 studies the Company is conducting, which doubled from three to six studies in 2021. SG&A expenses increased for the three months ended September 30, 2022 compared to the same period last year driven by Ionis' go-to-market activities for eplontersen, donidalorsen and olezarsen. SG&A expenses were lower for the nine months ended September 30, 2022 compared to the same period last year largely due to the substantial savings the Company achieved from integrating Akcea and restructuring its commercial operations in 2021.
Balance Sheet
As of September 30, 2022, Ionis had cash, cash equivalents and short-term investments of $2.0 billion, compared with $2.1 billion at December 31, 2021. Ionis' debt obligations and working capital did not change significantly from December 31, 2021 to September 30, 2022.
In October 2022, Ionis entered into a sale and leaseback transaction for several of its real estate assets. Under the agreement, Ionis will receive net proceeds of approximately $240 million plus full funding to expand the Company's R&D campus. The net proceeds reflect the Company's extinguishment of its mortgage debt for the related properties. Ionis' fourth quarter financial results will reflect the impact this transaction.
2022 Financial Guidance
The Company reaffirmed its full year 2022 guidance for total revenue, operating expenses and net loss, on a non-GAAP basis. The Company's 2022 operating expense guidance, compared to the prior year, includes increasing R&D expenses between 25% and 30% and consistent SG&A expenses. Ionis expects to recognize a substantial gain on the sale of its real estate assets in the fourth quarter. The gain will not impact the Company's non-GAAP results since the sale was non-recurring and not part of the Company's normal business operations.
The Company increased its full year 2022 cash and investments guidance to approximately $2.0 billion from the previous guidance of $1.7 billion.
"Our strong year-to-date results, including year-over-year revenue growth of nearly 20 percent, keep us on track to meet our 2022 P&L guidance," said Elizabeth L. Hougen, chief financial officer of Ionis. "Additionally, we recently bolstered our balance sheet when we unlocked net proceeds of approximately $240 million plus full funding to expand our R&D campus by capitalizing on the favorable life sciences real estate market and monetizing several of our facilities through a sale and leaseback transaction. As a result, we are increasing our 2022 cash and short-term investment guidance to approximately $2 billion."
Webcast
Management will host a conference call and webcast to discuss Ionis' third quarter 2022 results at 11:30 a.m. Eastern time on Wednesday, November 9, 2022. Interested parties may access the webcast here. A webcast replay will be available for a limited time at the same address. To access the Company's third quarter 2022 earnings slides click here.
About Ionis Pharmaceuticals, Inc.
For more than 30 years, Ionis has been the leader in RNA-targeted therapy, pioneering new markets and changing the standards of care with its novel antisense technology. Ionis currently has three marketed medicines and a premier late-stage pipeline highlighted by industry leading cardiovascular and neurological franchises. Our scientific innovation began and continues with the knowledge that sick people depend on us, which fuels our vision of becoming a leading, fully integrated biotechnology company.
To learn more about Ionis visit or follow us on Twitter @ionispharma.
Ionis' Forward-looking Statement
This press release includes forward-looking statements regarding Ionis' business, financial guidance and the therapeutic and commercial potential of SPINRAZA (nusinersen), TEGSEDI (inotersen), WAYLIVRA (volanesorsen), eplontersen, olezarsen, donidalorsen, ION363, pelacarsen, tofersen, Ionis' technologies and Ionis' other products in development. Any statement describing Ionis' goals, expectations, financial or other projections, intentions or beliefs is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to certain risks and uncertainties, including those related to the impact COVID-19 could have on our business, and including those inherent in the process of discovering, developing and commercializing medicines that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such medicines. Ionis' forward-looking statements also involve assumptions that, if they never materialize or prove correct, could cause its results to differ materially from those expressed or implied by such forward-looking statements. Although Ionis' forward-looking statements reflect the good faith judgment of its management, these statements are based only on facts and factors currently known by Ionis. As a result, you are cautioned not to rely on these forward-looking statements. These and other risks concerning Ionis' programs are described in additional detail in Ionis' annual report on Form 10-K for the year ended December 31, 2021, and most recent Form 10-Q, which are on file with the Securities and Exchange Commission. Copies of these and other documents are available from the Company.
In this press release, unless the context requires otherwise, "Ionis," "Company," "we," "our" and "us" all refer to Ionis Pharmaceuticals and its subsidiaries.
Ionis Pharmaceuticals® is a registered trademark of Ionis Pharmaceuticals, Inc. Akcea Therapeutics® is a registered trademark of Akcea Therapeutics, Inc. TEGSEDI® is a registered trademark of Akcea Therapeutics, Inc. WAYLIVRA® is a registered trademark of Akcea Therapeutics, Inc. SPINRAZA® is a registered trademark of Biogen.
Reconciliation of GAAP to Non-GAAP Basis
As illustrated in the Selected Financial Information in this press release, non-GAAP operating expenses, non-GAAP loss from operations, and non-GAAP net loss were adjusted from GAAP to exclude compensation expense related to equity awards and the related tax effects. Compensation expense related to equity awards are non-cash. In 2021 all non-GAAP amounts also excluded expenses related to the Akcea merger and restructured commercial operations. Expenses related to the Akcea merger and restructured commercial operations included: severance costs, retention costs and other costs related to commercial operations. Ionis has regularly reported non-GAAP measures for operating results as non-GAAP results. These measures are provided as supplementary information and are not a substitute for financial measures calculated in accordance with GAAP. Ionis reports these non-GAAP results to better enable financial statement users to assess and compare its historical performance and project its future operating results and cash flows. Further, the presentation of Ionis' non-GAAP results is consistent with how Ionis' management internally evaluates the performance of its operations.
Ionis' financial results for the nine months ended September 30, 2022 reflected the cost-sharing provisions related to its collaboration with AstraZeneca to develop and commercialize eplontersen for the treatment of ATTR. Under the terms of the collaboration agreement, AstraZeneca is paying 55 percent of the costs associated with the ongoing global Phase 3 development program. Because Ionis is leading and conducting the Phase 3 development program, Ionis is recognizing the 55 percent of cost-share funding AstraZeneca is responsible for, net of Ionis' share of AstraZeneca's development expenses, as R&D revenue in the same period Ionis incurs the related development expenses. For the nine months ended September 30, 2022 Ionis earned $55 million in joint development revenue under this collaboration.
Because AstraZeneca is responsible for the majority of the medical affairs and commercial costs in the U.S. and all costs associated with bringing eplontersen to market outside the U.S., Ionis is recognizing cost-share funding it receives from AstraZeneca related to these activities as a reduction of its medical affairs (R&D expenses) and commercialization expenses (SG&A expenses). For the nine months ended September 30, 2022 Ionis recognized $1.4 million and $1.5 million of medical affairs expenses and commercialization expenses for eplontersen, respectively, net of cost-share funding from AstraZeneca. Ionis expects its medical affairs and commercialization expenses to increase as this collaboration progresses.
2022 Pipeline Milestones(1)
SOURCE Ionis Pharmaceuticals, Inc.
Financial StatementPriority ReviewAcquisitionCollaborate
09 Nov 2022
Advanced key priorities: initiated manufacturing infrastructure project to support growth
Increased 2022 cash and investments guidance
CARLSBAD, Calif., Nov. 9, 2022 /PRNewswire/ -- Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) (the "Company") today reported financial results for the third quarter of 2022. Financial results are summarized below:
Financial Highlights
Revenue increased 20% for the third quarter of 2022 and 18% on a year-to-date basis compared to the same periods last year driven by significant partner payments earned across multiple programs
Non-GAAP operating expenses increased 5% for the third quarter of 2022 and 13% on a year-to-date basis compared to the same periods last year driven by advancing Phase 3 pipeline
Entered into a long-term lease in October 2022 to construct a new manufacturing facility supporting continued growth
Entered into a sale-leaseback transaction in October 2022 for several real estate assets, generating net proceeds of $240 million plus full funding to expand R&D campus
Reaffirmed 2022 P&L guidance; increased cash and investments guidance to approximately $2.0 billion
Late-Stage Pipeline Highlights
Presented positive data from the Phase 3 NEURO-TTRansform study of eplontersen in patients with polyneuropathy caused by hereditary TTR amyloidosis; on track to New Drug Application this year
Expanded enrollment in the Phase 3 CARDIO-TTRansform study of eplontersen in patients with ATTR cardiomyopathy; data still expected first half of 2025
NDA for tofersen was accepted and granted priority review by the FDA; Prescription Drug User Fee Act date of April 25, 2023
Initiated CORE2, a confirmatory Phase 3 study of olezarsen in patients with severe hypertriglyceridemia (SHTG)
Initiated ESSENCE, a supporting Phase 3 study of olezarsen in patients with SHTG or hypertriglyceridemia and cardiovascular disease
Mid-Stage Pipeline Highlights
GSK presented positive end of study data from the Phase 2b B-Clear study of bepirovirsen demonstrating potential for functional cures in patients with chronic hepatitis B; GSK plans to advance bepirovirsen into Phase 3 development in the first half of 2023
Presented positive data from the Phase 2 study of IONIS-FB-LRx in patients with immunoglobulin A nephropathy; Roche plans to advance IONIS-FB-LRx into Phase 3 development in the first half of 2023
Bayer presented positive data from the Phase 2b study of fesomersen in patients with end-stage renal disease; Ionis regained rights to fesomersen from Bayer and is assessing next steps
Roche presented the Phase 2 GENERATION HD2 study design of tominersen in Huntington's disease patients; Roche plans to begin enrollment in early 2023
Reported ION449 (AZD8233) targeting PCSK9 met the primary endpoint in Phase 2b SOLANO study in patients with hypercholesterolemia; based on pre-specified efficacy criteria, AstraZeneca is not advancing ION449
"We have made significant progress on our key priorities this year, building our commercial pipeline, delivering an abundance of new medicines to the market and expanding and diversifying our technology. We delivered positive data from eight key programs, positioning us to potentially add two new marketed medicines to our portfolio and expand our rich Phase 3 pipeline to eight medicines next year. Additionally, we have recently begun work on a manufacturing facility to support our pipeline growth," said Brett P. Monia, Ph.D., chief executive officer of Ionis. "As we advance our near-term opportunities, including filing the NDA this year for eplontersen, and expanding our rich late-stage pipeline, we are well positioned to drive increasing value for all stakeholders."
Third Quarter 2022 Financial Results
Revenue
Ionis' revenue was comprised of the following:
Total revenue for the three and nine months ended September 30, 2022 increased 20 percent and 18 percent compared to the same periods last year, respectively. The increase was driven by significant payments Ionis earned across multiple partnered programs. R&D revenue for the nine months ended September 30, 2022 included $85 million from Biogen for advancing several neurology disease programs, $63 million from Roche for licensing and advancing IONIS-FB-LRx and $55 million from AstraZeneca for its share of the global Phase 3 development costs for eplontersen.
Commercial revenue for the three and nine months ended September 30, 2022 decreased 15 percent and 13 percent compared to the same periods last year, respectively. SPINRAZA royalties for the three and nine months ended September 30, 2022 decreased 7 percent and 12 percent compared to the same periods last year, respectively. In the U.S., SPINRAZA sales were flat in the first nine months of 2022 compared to the same period last year. Outside of the U.S. SPINRAZA royalties were lower due to lower SPINRAZA product sales primarily due to decreased pricing, foreign currency exchange and competition. TEGSEDI and WAYLIVRA revenue was also lower due to the shift to distribution fees in 2021.
Operating Expenses
Ionis' non-GAAP operating expenses increased for the three and nine months ended September 30, 2022 compared to the same periods in 2021, in line with expectations. For both periods, higher R&D expenses were driven by the expanded number of Phase 3 studies the Company is conducting, which doubled from three to six studies in 2021. SG&A expenses increased for the three months ended September 30, 2022 compared to the same period last year driven by Ionis' go-to-market activities for eplontersen, donidalorsen and olezarsen. SG&A expenses were lower for the nine months ended September 30, 2022 compared to the same period last year largely due to the substantial savings the Company achieved from integrating Akcea and restructuring its commercial operations in 2021.
Balance Sheet
As of September 30, 2022, Ionis had cash, cash equivalents and short-term investments of $2.0 billion, compared with $2.1 billion at December 31, 2021. Ionis' debt obligations and working capital did not change significantly from December 31, 2021 to September 30, 2022.
In October 2022, Ionis entered into a sale and leaseback transaction for several of its real estate assets. Under the agreement, Ionis will receive net proceeds of approximately $240 million plus full funding to expand the Company's R&D campus. The net proceeds reflect the Company's extinguishment of its mortgage debt for the related properties. Ionis' fourth quarter financial results will reflect the impact this transaction.
2022 Financial Guidance
The Company reaffirmed its full year 2022 guidance for total revenue, operating expenses and net loss, on a non-GAAP basis. The Company's 2022 operating expense guidance, compared to the prior year, includes increasing R&D expenses between 25% and 30% and consistent SG&A expenses. Ionis expects to recognize a substantial gain on the sale of its real estate assets in the fourth quarter. The gain will not impact the Company's non-GAAP results since the sale was non-recurring and not part of the Company's normal business operations.
The Company increased its full year 2022 cash and investments guidance to approximately $2.0 billion from the previous guidance of $1.7 billion.
"Our strong year-to-date results, including year-over-year revenue growth of nearly 20 percent, keep us on track to meet our 2022 P&L guidance," said Elizabeth L. Hougen, chief financial officer of Ionis. "Additionally, we recently bolstered our balance sheet when we unlocked net proceeds of approximately $240 million plus full funding to expand our R&D campus by capitalizing on the favorable life sciences real estate market and monetizing several of our facilities through a sale and leaseback transaction. As a result, we are increasing our 2022 cash and short-term investment guidance to approximately $2 billion."
Webcast
Management will host a conference call and webcast to discuss Ionis' third quarter 2022 results at 11:30 a.m. Eastern time on Wednesday, November 9, 2022. Interested parties may access the webcast here. A webcast replay will be available for a limited time at the same address. To access the Company's third quarter 2022 earnings slides click here.
About Ionis Pharmaceuticals, Inc.
For more than 30 years, Ionis has been the leader in RNA-targeted therapy, pioneering new markets and changing the standards of care with its novel antisense technology. Ionis currently has three marketed medicines and a premier late-stage pipeline highlighted by industry leading cardiovascular and neurological franchises. Our scientific innovation began and continues with the knowledge that sick people depend on us, which fuels our vision of becoming a leading, fully integrated biotechnology company.
To learn more about Ionis visit or follow us on Twitter @ionispharma.
Ionis' Forward-looking Statement
This press release includes forward-looking statements regarding Ionis' business, financial guidance and the therapeutic and commercial potential of SPINRAZA (nusinersen), TEGSEDI (inotersen), WAYLIVRA (volanesorsen), eplontersen, olezarsen, donidalorsen, ION363, pelacarsen, tofersen, Ionis' technologies and Ionis' other products in development. Any statement describing Ionis' goals, expectations, financial or other projections, intentions or beliefs is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to certain risks and uncertainties, including those related to the impact COVID-19 could have on our business, and including those inherent in the process of discovering, developing and commercializing medicines that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such medicines. Ionis' forward-looking statements also involve assumptions that, if they never materialize or prove correct, could cause its results to differ materially from those expressed or implied by such forward-looking statements. Although Ionis' forward-looking statements reflect the good faith judgment of its management, these statements are based only on facts and factors currently known by Ionis. As a result, you are cautioned not to rely on these forward-looking statements. These and other risks concerning Ionis' programs are described in additional detail in Ionis' annual report on Form 10-K for the year ended December 31, 2021, and most recent Form 10-Q, which are on the Securities and Exchange Commission. Copies of these and other documents are available from the Company.
In this press release, unless the context requires otherwise, "Ionis," "Company," "we," "our" and "us" all refer to Ionis Pharmaceuticals and its subsidiaries.
Ionis Pharmaceuticals® is a registered trademark of Ionis Pharmaceuticals, Inc. Akcea Therapeutics® is a registered trademark of Akcea Therapeutics, Inc. TEGSEDI® is a registered trademark of Akcea Therapeutics, Inc. WAYLIVRA® is a registered trademark of Akcea Therapeutics, Inc. SPINRAZA® is a registered trademark of Biogen.
Reconciliation of GAAP to Non-GAAP Basis
As illustrated in the Selected Financial Information in this press release, non-GAAP operating expenses, non-GAAP loss from operations, and non-GAAP net loss were adjusted from GAAP to exclude compensation expense related to equity awards and the related tax effects. Compensation expense related to equity awards are non-cash. In 2021 all non-GAAP amounts also excluded expenses related to the Akcea merger and restructured commercial operations. Expenses related to the Akcea merger and restructured commercial operations included: severance costs, retention costs and other costs related to commercial operations. Ionis has regularly reported non-GAAP measures for operating results as non-GAAP results. These measures are provided as supplementary information and are not a substitute for financial measures calculated in accordance with GAAP. Ionis reports these non-GAAP results to better enable financial statement users to assess and compare its historical performance and project its future operating results and cash flows. Further, the presentation of Ionis' non-GAAP results is consistent with how Ionis' management internally evaluates the performance of its operations.
Ionis' financial results for the nine months ended September 30, 2022 reflected the cost-sharing provisions related to its collaboration with AstraZeneca to develop and commercialize eplontersen for the treatment of ATTR. Under the terms of the collaboration agreement, AstraZeneca is paying 55 percent of the costs associated with the ongoing global Phase 3 development program. Because Ionis is leading and conducting the Phase 3 development program, Ionis is recognizing the 55 percent of cost-share funding AstraZeneca is responsible for, net of Ionis' share of AstraZeneca's development expenses, as R&D revenue in the same period Ionis incurs the related development expenses. For the nine months ended September 30, 2022 Ionis earned $55 million in joint development revenue under this collaboration.
Because AstraZeneca is responsible for the majority of the medical affairs and commercial costs in the U.S. and all costs associated with bringing eplontersen to market outside the U.S., Ionis is recognizing cost-share funding it receives from AstraZeneca related to these activities as a reduction of its medical affairs (R&D expenses) and commercialization expenses (SG&A expenses). For the nine months ended September 30, 2022 Ionis recognized $1.4 million and $1.5 million of medical affairs expenses and commercialization expenses for eplontersen, respectively, net of cost-share funding from AstraZeneca. Ionis expects its medical affairs and commercialization expenses to increase as this collaboration progresses.
2022 Pipeline Milestones(1)
View original content to download multimedia:
SOURCE Ionis Pharmaceuticals, Inc.
Company Codes: NASDAQ-NMS:IONS
Financial StatementPriority ReviewAcquisitionCollaborate
100 Deals associated with Fesomersen
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Thrombosis | Phase 2 | United States | 04 Sep 2020 | |
| Thrombosis | Phase 2 | Japan | 04 Sep 2020 | |
| Thrombosis | Phase 2 | Belgium | 04 Sep 2020 | |
| Thrombosis | Phase 2 | Bulgaria | 04 Sep 2020 | |
| Thrombosis | Phase 2 | Canada | 04 Sep 2020 | |
| Thrombosis | Phase 2 | Czechia | 04 Sep 2020 | |
| Thrombosis | Phase 2 | Germany | 04 Sep 2020 | |
| Thrombosis | Phase 2 | Greece | 04 Sep 2020 | |
| Thrombosis | Phase 2 | Hungary | 04 Sep 2020 | |
| Thrombosis | Phase 2 | Latvia | 04 Sep 2020 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
NCT04534114 (Pubmed) Manual | Phase 2 | Kidney Failure, Chronic Protein Expression Markers | 307 | owubpvybmo(waberpybeg) = teurndlebz rishlhsxtj (gwjtbrlxdq ) View more | Negative | 01 Mar 2024 | |
owubpvybmo(waberpybeg) = fahboiybqy rishlhsxtj (gwjtbrlxdq ) View more | |||||||
Phase 2 | 307 | (Fesomersen 40 mg) | ohlsybrkkq = brhwdqqpto dkrnnufujo (fhbfrgdpev, tyucksxkfk - wnhqqhkelm) View more | - | 03 Jul 2023 | ||
(Fesomersen 80 mg) | ohlsybrkkq = pecqoagqep dkrnnufujo (fhbfrgdpev, zylrcxhevq - alvcggmtyc) View more | ||||||
Phase 2 | FXI levels | 307 | mvhgjkntzp(levdstaejv) = dgfzqrgxoz gdiqnavoeo (bmvswrmjil ) View more | Positive | 03 Nov 2022 | ||
mvhgjkntzp(levdstaejv) = qkboizjagz gdiqnavoeo (bmvswrmjil ) View more | |||||||
Phase 2 | 307 | tgkrqpvftb(ucgywhybyl) = fesomersen achieved its primary outcome measure of no increase in incidence of major bleeding and clinically relevant non-major bleeding as compared to placebo. zzxbeshiut (cjybmqtdnz ) View more | Positive | 28 Jul 2022 | |||
Placebo |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free

