Request Demo
Last update 24 Jan 2026
Dexpanthenol
Last update 24 Jan 2026
Overview
Basic Info
Drug Type Small molecule drug |
Synonyms (+)-Panthenol, (2R)-2,4-dihydroxy-N-(3-hydroxypropyl)-3,3-dimethylbutanamide, Bepanthene + [27] |
Target |
Action agonists |
Mechanism mAChRs agonists(Muscarinic acetylcholine receptor agonists) |
Therapeutic Areas |
Active Indication |
Inactive Indication |
Originator Organization |
Active Organization |
License Organization |
Drug Highest PhaseApproved |
First Approval Date Japan (20 Mar 1959), |
Regulation- |
Login to view timeline
Structure/Sequence
Molecular FormulaC9H19NO4 |
InChIKeySNPLKNRPJHDVJA-ZETCQYMHSA-N |
CAS Registry81-13-0 |
Related
41
Clinical Trials associated with DexpanthenolCTIS2023-510522-32-00
MucPreDex: Radiation-induced MUCositis PREvention using DEXpanthenol mouth rinse
Start Date29 Aug 2025 |
Sponsor / Collaborator- |
NCT06822608
Evaluation of a Fixed Combination of Dexpanthenol and Hyaluronic Acid Preservative-free Eye Drops on Corneal Epithelialization After Trans Epithelial PRK: a Prospective, Contralateral, Randomized, Double-blind, Placebo-controlled Trial
The aim of this prospective, contralateral, randomized, double-blind, placebo-controlled study is the evaluation of the performance of a fixed combination of dexpanthenol and hyaluronic acid preservative-free eye artificial tears eye drops, in corneal wound healing after trans epithelial photorefractive keratectomy. One eye of each patient, randomly determined, will be assigned to receive artificial tears containing a combination of dexpanthenol-hyaluronic acid (study group) while the fellow eye will receive Placebo / identical combination containing the same ingredients and hyaluronic acid without dexpanthenol (control group). The drops will be applied hourly postoperatively, starting one hour after the surgery. All subjects will be treated until the day of complete reepithelialization or a maximum of 7 days postoperatively. Researchers will compare the epithelialization between the two eyes. The primary endpoint is the time to reepithelialization.The secondary endpoints are the intra-individual differences measured in each eye in terms of epithelial defect size in each visit, subjective evaluation of pain (discomfort) and subjective evaluation of vision.
Start Date01 Feb 2025 |
Sponsor / Collaborator |
IRCT20240809062706N2
The effect of topical dexpanthenol cream on the prevention of striae in primigravida women: A double-blind randomized controlled trial
Start Date24 Sep 2024 |
Sponsor / Collaborator |
100 Clinical Results associated with Dexpanthenol
Login to view more data
100 Translational Medicine associated with Dexpanthenol
Login to view more data
100 Patents (Medical) associated with Dexpanthenol
Login to view more data
879
Literatures (Medical) associated with Dexpanthenol01 Jun 2026·International Journal of Pharmaceutics-X
3D printed system using ex vivo porcine eye globes to investigate intracorneal absorption of dexpanthenol from contact lens care solution and eye drops
Article
Author: Santer, Verena ; El Guamra, Adyl-Michaёl ; Lamrani, Mouad ; Kalia, Yogeshvar N ; Kawaguchi, Tohru
Reduction of the use of animal models in research is encouraged for the sake of animal wellbeing. However, available in vitro models in the specific case of topical ocular delivery/penetration studies are often oversimplified by the use of excised corneal or scleral tissue and the frequent lack of dynamic barriers such as the lacrimal outwash. This is why we have developed our novel ex vivo porcine eye globe 3D printed laboratory setup with simulated tear flow, using enucleated porcine eyes. This setup was employed to investigate the penetration of a common topical formulation excipient Dexpanthenol (Dxp). First, Dxp deposition in soft contact lenses following usage of SOLOCARE AQUA® care solution was quantified by UHPLC-MS/MS. The subsequent penetration into corneal tissue and aqueous humour of Dxp from SOLOCARE AQUA® care solution treated contact lenses was compared to that following application of eye drop solutions Bepanthen® and Siccaprotect®, containing equivalent Dxp concentrations. The results showed that the Dxp concentration in the anterior segment was three-fold higher after application of the Dxp-containing eye drops as compared to contact lens application. Given that Dxp uptake was greater following the application of the marketed eye drops, this confirmed the safety of the Dxp-containing contact lens care solution. This research demonstrates how topical delivery studies on the ocular surface can be simulated in our novel ex vivo porcine eye globe model without the need to sacrifice laboratory animals.
01 Dec 2025·MOLECULAR BIOLOGY REPORTS
The pantothenic acid derivative dexpanthenol ameliorated doxorubicin-induced neurotoxicity via regulating AKT/CREB/BDNF and AKT/NRF2 signaling pathways
Article
Author: Savran, Mehtap ; Ozmen, Ozlem ; Imeci, Orhan ; Asci, Halil ; Unlu, Melike Dogan
BACKGROUND:
Doxorubicin (Dox)-induced neurotoxicity is a well-documented side effect of chemotherapy. Dexpanthenol (Dex), an analog of vitamin B5, has shown protective properties. This study aimed to explore the molecular mechanisms by which Dex mitigates Dox-induced neurotoxicity, particularly through the protein kinase B (AKT)/cyclic AMP-response element-binding protein (CREB)/brain-derived neurotrophic factor (BDNF) pathway and nuclear factor erythroid 2-related factor 2 (NRF2) signaling.
METHODS AND RESULTS:
The experiment was conducted using four groups: control, Dex, Dox, and Dox + Dex, comprising a total of 32 female Wistar Albino rats. After two weeks of treatment, the rats were euthanized, and brain and cerebellum tissues were collected for analysis. Biochemical analysis was performed spectrophotometrically to assess oxidative stress parameters, while histological and immunostaining analyses focused on nuclear factor kappa B (NF-κB) and inducible nitric oxide synthase (iNOS) immunoexpressions. Genetic analysis of AKT, CREB, BDNF, and NRF2 gene expressions was conducted using real-time polymerase chain reaction. Histopathological evaluation of the Dox group revealed hyperemia, microhemorrhage, neuronal damage, and neuronophagia. Additionally, an increase in caspase-3, tumor necrosis factor-alpha, NF-κB, and iNOS immunoexpressions were observed, along with elevated total oxidant status and oxidative stress index. A decrease in AKT, CREB, BDNF, and NRF2 gene expressions accompanied these changes. Dex treatment significantly reversed these pathological findings, effectively protecting the brain from Dox-induced neuronal damage.
CONCLUSION:
In conclusion, Dex may provide neuroprotection in female rats with Dox-induced neurotoxicity by activating the CREB/BDNF pathway and reducing oxidative stress through AKT-mediated NRF2 synthesis. Further detailed studies exploring additional pathways are required to incorporate Dex into cancer treatment protocols and minimize side effects.
01 Nov 2025·METABOLIC ENGINEERING
De novo biosynthesis of D-panthenol in engineered E. coli with rationally designed L-homoserine decarboxylase
Article
Author: Ren, Jie ; Liu, Feixia ; Yu, Bo ; Zheng, Peng ; Han, Xinhao ; Zheng, Jie
D-panthenol is a compound of significant importance in the pharmaceutical, cosmetic, and nutraceutical sectors, attributed to its remarkable moisturizing, anti-inflammatory, and tissue repair properties. Traditional chemical synthesis encounters several challenges, including the generation of toxic by-products, low enantiomeric excess, and expensive purification processes. To date, complete biosynthesis of D-panthenol solely from glucose has seldom been documented. In this study, we have developed a new fermentative route to produce D-panthenol. The pathway incorporates previously unreported reaction of decarboxylating L-homoserine to 3-amino-1-propanol, which is achieved by rational design of a novel tyrosine decarboxylase mutant, informed by structural and mechanistic insights into enzymes acting on sterically similar substrates. The next enzyme facilitating the condensation of 3-amino-1-propanol with D-pantoate for D-panthenol formation was identified through a comprehensive screening of natural D-pantothenate synthetases. The artificial pathway was functionally expressed in a minimally engineered E. coli strain, resulting in the de novo production of D-panthenol from glucose. This research highlights a demonstration of an unnatural enzymatic synthesis process for D-panthenol. With further strain and process engineering, this new approach could be a promising way to produce D-panthenol biologically.
5
News (Medical) associated with Dexpanthenol23 Aug 2024
New Delhi: In a significant move aimed at safeguarding public health, the Indian government has banned 156 fixed-dose combination (FDC) drugs, including a variety of antibiotics, painkillers, multivitamins, and medications for treating fever and hypertension. This decision marks the largest crackdown on FDCs since 2016, when 344 such drugs were prohibited. The Union Ministry of Health and Family Welfare issued a gazette notification on Thursday, officially prohibiting the manufacture, sale, and distribution of the banned FDCs. The ban was based on the recommendations of an expert panel that reviewed a total of 324 FDCs.
Impact on Pharmaceutical Industry
The banned FDCs include popular combinations such as mefenamic acid and paracetamol injections, commonly used for pain relief and fever, and omeprazole magnesium with dicyclomine HCl, used to treat abdominal pain. The ban is expected to impact major pharmaceutical companies, including Sun Pharmaceuticals, Cipla, Dr. Reddy's Laboratories, Torrent Pharmaceuticals, and Alkem Laboratories.
Fixed Dose Combinations
Drug Banned List
Here is a table listing all 156 banned Fixed Dose Combinations (FDCs)
SN
Fixed Dose Combination (FDC)
1
Amylase + Protease + Glucoamylase + Pectinase + Alpha Galactosidase + Lactase + Beta-Gluconase + Cellulase + Lipase + Bromelain + Xylanase + Hemicellulase + Malt diastase + Invertase + Papain
2
Antimony Potassium Tartrate + Dried Ferrous Sulphate
3
Benfotiamine + Silymarin + L-Ornithine L-aspartate + Sodium Selenite + Folic acid + Pyridoxine hydrochloride
4
Bismuth Ammonium Citrate + Papain
5
Cyproheptadine HCl + Thiamine HCl + Riboflavine + Pyridoxine HCl + Niacinamide
6
Cyproheptadine Hydrochloride + Tricholine Citrate + Thiamine Hydrochloride + Riboflavine + Pyridoxine Hydrochloride
7
Rabeprazole Sodium (As enteric coated tablet) + Clidinium Bromide + Dicyclomine HCl + Chlordiazepoxide
8
Fungal Diastase + Papain + Nux vomica Tincture + Cardamom Tincture + Casein Hydrolysed + Alcohol
9
Mefenamic Acid + Paracetamol Injection
10
Omeprazole Magnesium + Dicyclomine HCl
11
S-adenosyl methionine + Metadoxine + Ursodeoxycholicacid BP + L-Methylfolate Calcium eq. to L- Methylfolate + Choline bitartratee + Silymarin + L-ornithine Laspartate + Inositol + Taurine
12
Silymarin + Thiamine Mononitrate + Riboflavin + Pyridoxine HCl + Niacinamide + Calcium pantothenate + Vitamin B12
13
Silymarin + Pyridoxine HCl + Cyanocobalamin + Niacinamide + Folic Acid
14
Silymarin + Vitamin B6 + Vitamin B12 + Niacinamide + Folic acid + Tricholine Citrate
15
Sodium Citrate + Citric Acid Monohydrate Flavored with Cardamom Oil, Caraway Oil, Cinnamon Oil, Clove Oil, Ginger Oil + Alcohol
16
Sucralfate + Acelofenac
17
Sucralfate + Domperidone + Dimethicone
18
Sucralfate + Domperidone
19
Tincture Ipecacuanha + Tincture Urgenia + Camphorated Opium Tincture + Aromatic Spirit of Ammonia + Chloroform + Alcohol
20
Ursodeoxycholic Acid + Metformin HCl
21
Weak Ginger tincture + Aromatic Spirit of Ammonia + Peppermint Spirit + Chloroform + Sodium Bicarbonate + Compound Cardamom + Alcohol
22
Sucralfate + Pantoprazole Sodium + Zinc Gluconate + Light Magnesium Carbonate
23
Aloe + Vitamin E Soap
24
Povidone Iodine+ Metronidazole + Aloe
25
Azelaic acid + Tea Tree Oil + Salicylic acid + Allantoin + Zinc oxide + Aloe vera + Jojoba oil + Vitamin E + Soap noodles
26
Azithromycin + Adapalene
27
Calamine + Aloes + Allantoin
28
Calamine + Diphenhydramine Hydrochloride + Aloe + Glycerine + Camphor
29
Chlorphenesin + Zinc oxide + Starch
30
Clindamycin Phosphate + Zinc acetate
31
Gamma Benzene Hexachloride + Benzocaine
32
Glucosamine hydrochloride + Diacerein + Menthol + Camphor + Capsaicin
33
Hydroxyquinone 2.0%w/w + Octyl Methoxycinnamate 5.0% w/w + Oxybenzone 30 % w/w
34
Ketoconazole +Zinc Pyrithione +D-Panthenol +Tea Tree Oil +Aloes
35
Ketoconazole +Aloe vera+ Vitamin A Acetate
36
Ketoconazole +Aloes + ZPTO
37
Kojic Acid +Arbutin +Octinoxate +Vitamin E + Mulberry
38
Lornoxicam +capsaicin +menthol+ camphor
39
Lornoxicam + Thiocolchicoside +Oleum Lini +Menthol + Methyl salicylate
40
Menthol + Aloe vera Topical Spray
41
Menthol +Lignocaine HCl +Aloe vera gel +Clotrimazole +Diphenhydramine
42
Miconazole nitrate + Gentamicin + Fluocinolone Acetonide +Zinc Sulphate
43
Miconazole +Tinidazole
44
Minoxidil +Aminexil+ Alcohol
45
Minoxidil +Azelaic acid + saw palmetto
46
Minoxidil +Aminexil
47
Pine Bark extract+ Kojic acid +Sodium Ascorbyl Phosphate
48
Povidone Iodine +Tinidazole +Zinc sulphate
49
Povidone Iodine + Ornidazole + Dexpanthenol
50
Salicyclic acid +Aloe vera+ Allantoin +D-Panthenol
51
Silver sulphadiazine +Chlorhexidine Gluconate solution +Allantoin + Aloe vera gel +Vitamin E acetate
52
Sodium salicylate + Zinc gluconate + Pyridoxine HCl
53
Tetracycline + Colistin Sulphate
54
Clomiphene +Ubidecarenone
55
Combikit of Clomiphene Citrate + Estradiol Valerate
56
Flavoxate HCl +Ofloxacin
57
Clomiphene Citrate +N-Acetylcysteine
58
Primerose Oil +Cod liver oil
59
Sildenafil Citrate +Papaverine +L-Arginine
60
Tranexamic acid +Mefenamic acid + Vitamin K1
61
Divalproex Sodium +Oxcarbazepine
62
Divalproex Sodium + Levetiracitam
63
Ergotamine tartrate +Caffeine + Paracetamol +Prochlorperazine maleate
64
Piracetam + Ginkgo biloba extracts + Vinpocetin
65
Ginkgo biloba + methylcobalamin
66
Ginkgo biloba + methylcobalamin + Alpha lipoic acid +Pyridoxine HCl
67
Ginseng Extract +Dried extract of Ginkgo Biloba
68
Meclizine HCl+ Paracetamol + Caffeine
69
Nicergoline + Vinpocetine
70
Gamma Linolenic Acid + Methylcobalamin
71
Beclomethasone Dipropionate + Neomycin Sulphate + Clotrimazole + Lignocaine HCl
72
Boric acid+ Phenylephrine HCl + Naphazoline Nitrate + Menthol + Camphor
73
Naphazoline HCl + Chlorpheniramine Maleate + Zinc Sulphate + Hydroxy Propyl Methyl Cellulose
74
Chlorpheniramine Maleate + Naphazoline HCl + Zinc Sulphate + Sodium Chloride + Hydroxy Propyl Methyl Cellulose
75
Chlorpheniramine Maleate + Naphazoline HCl + Hydroxy Propyl Methyl Cellulose
76
Chlorpheniramine Maleate + Sodium Chloride + Boric Acid + Tetrahydrozoline HCl
77
Chlorpheniramine Maleate + Phenylephrine HCl + Antipyrine
78
Ketorolac Tromethamine + Chlorpheniramine Maleate + Phenylephrine HCl + Hydroxy Propyl Methyl Cellulose
79
Ketorolac Tromethamine + Flurometholone
80
Naphazoline HCl + Zinc Sulphate + Boric Acid + Sodium Chloride+ Chlorpheniramine Maleate
81
Naphazoline HCl + Hydroxy Propyl Methyl Cellulose + Boric Acid + Borax + Menthol + Camphor
82
Naphazoline HCl + Hydroxy Propyl Methyl Cellulose + Chlorpheniramine Maleate
SN
Fixed Dose Combination (FDC)
83
Diphenhydramine Hydrochloride + Phenylephrine Hydrochloride + Menthol + Camphor
84
Diphenhydramine Hydrochloride + Phenylephrine Hydrochloride + Sodium Chloride + Hydroxy Propyl Methyl Cellulose
85
Diphenhydramine Hydrochloride + Phenylephrine Hydrochloride + Sodium Chloride + Methylparaben
86
Methenamine + Sodium Benzoate + Benzyl Alcohol
87
Paracetamol + Phenylephrine Hydrochloride + Chlorpheniramine Maleate
88
Paracetamol + Phenylephrine Hydrochloride + Chlorpheniramine Maleate + Caffeine
89
Propoxyphene + Chlorpheniramine Maleate
90
Propoxyphene + Phenylephrine Hydrochloride + Chlorpheniramine Maleate
91
Propoxyphene + Diphenhydramine Hydrochloride + Phenylephrine Hydrochloride
92
Sodium Benzoate + Sodium Citrate + Citric Acid
93
Vitamin A Palmitate + Vitamin C + Vitamin E + Vitamin B6 + Vitamin B12
94
Vitamin A Palmitate + Vitamin C + Vitamin E + Niacinamide
95
Vitamin B6 + Vitamin B12 + Vitamin C
96
Vitamin B6 + Vitamin B12 + Niacinamide
97
Vitamin B12 + Vitamin B6 + Vitamin C + Folic Acid
98
Vitamin B12 + Vitamin B6 + Niacinamide + Folic Acid
99
Vitamin C + Vitamin E + Vitamin A + Niacinamide
100
Vitamin D + Calcium + Magnesium
101
Vitamin D + Calcium + Magnesium + Zinc
102
Calcium + Vitamin D + Vitamin K
103
Calcium + Vitamin D + Magnesium + Zinc
104
Calcium + Vitamin D + Vitamin K + Magnesium
105
Calcium + Vitamin D + Vitamin K + Magnesium + Zinc
106
Vitamin E + Vitamin A + Vitamin C + Niacinamide
107
Vitamin E + Vitamin A + Vitamin C + Folic Acid
108
Vitamin E + Vitamin A + Vitamin C + Biotin
109
Vitamin E + Vitamin A + Vitamin C + Vitamin B12
110
Vitamin E + Vitamin C + Folic Acid
111
Vitamin E + Vitamin C + Biotin
112
Vitamin E + Vitamin C + Vitamin B12
113
Vitamin E + Vitamin C + Zinc
114
Calcium + Vitamin D + Omega-3 Fatty Acids
115
Calcium + Vitamin D + Omega-3 Fatty Acids + Vitamin K
116
Calcium + Vitamin D + Omega-3 Fatty Acids + Vitamin K + Magnesium
117
Calcium + Vitamin D + Omega-3 Fatty Acids + Vitamin K + Zinc
118
Vitamin A + Vitamin C + Vitamin E + Beta-Carotene
119
Vitamin A + Vitamin C + Vitamin E + Beta-Carotene + Zinc
120
Vitamin C + Vitamin E + Beta-Carotene
121
Vitamin C + Vitamin E + Beta-Carotene + Zinc
122
Vitamin C + Vitamin E + Selenium
123
Vitamin D + Calcium + Vitamin K + Omega-3 Fatty Acids
124
Vitamin D + Calcium + Vitamin K + Omega-3 Fatty Acids + Magnesium
125
Vitamin D + Calcium + Vitamin K + Omega-3 Fatty Acids + Zinc
126
Vitamin D + Calcium + Vitamin K + Omega-3 Fatty Acids + Biotin
127
Vitamin D + Calcium + Omega-3 Fatty Acids + Vitamin K + Selenium
128
Vitamin E + Vitamin A + Vitamin C + Zinc
129
Vitamin E + Vitamin A + Vitamin C + Selenium
130
Vitamin E + Vitamin A + Vitamin C + Biotin
131
Vitamin E + Vitamin A + Vitamin C + Folic Acid + Niacinamide
132
Vitamin E + Vitamin C + Beta-Carotene + Zinc
133
Vitamin C + Vitamin E + Biotin + Folic Acid
134
Vitamin C + Vitamin E + Beta-Carotene + Folic Acid
135
Vitamin C + Vitamin E + Beta-Carotene + Selenium
136
Vitamin E + Vitamin C + Beta-Carotene + Biotin
137
Vitamin D + Calcium + Omega-3 Fatty Acids + Vitamin K + Biotin
138
Vitamin D + Calcium + Omega-3 Fatty Acids + Vitamin K + Selenium
139
Vitamin D + Calcium + Omega-3 Fatty Acids + Vitamin K + Vitamin B12
140
Vitamin D + Calcium + Omega-3 Fatty Acids + Vitamin K + Vitamin B6
141
Vitamin D + Calcium + Omega-3 Fatty Acids + Vitamin K + Folate
142
Calcium + Vitamin D + Magnesium + Vitamin K + Omega-3 Fatty Acids
143
Calcium + Vitamin D + Magnesium + Vitamin K + Biotin
144
Calcium + Vitamin D + Magnesium + Vitamin K + Selenium
145
Calcium + Vitamin D + Magnesium + Vitamin K + Vitamin B12
146
Calcium + Vitamin D + Magnesium + Vitamin K + Folate
147
Calcium + Vitamin D + Vitamin K + Omega-3 Fatty Acids + Vitamin B6
148
Calcium + Vitamin D + Vitamin K + Omega-3 Fatty Acids + Vitamin B12
149
Calcium + Vitamin D + Vitamin K + Omega-3 Fatty Acids + Folic Acid
150
Calcium + Vitamin D + Vitamin K + Omega-3 Fatty Acids + Biotin
151
Calcium + Vitamin D + Vitamin K + Omega-3 Fatty Acids + Selenium
152
Vitamin A + Vitamin C + Vitamin E + Omega-3 Fatty Acids
153
Vitamin A + Vitamin C + Vitamin E + Omega-3 Fatty Acids + Zinc
154
Vitamin A + Vitamin C + Vitamin E + Omega-3 Fatty Acids + Biotin
155
Vitamin A + Vitamin C + Vitamin E + Omega-3 Fatty Acids + Selenium
156
Vitamin A + Vitamin C + Vitamin E + Omega-3 Fatty Acids + Vitamin B12

Drug Approval
27 Feb 2023
Leverkusen, February 28, 2023
– The Bayer Group achieved strong growth last year, posting significantly higher sales and earnings. “2022 was a very successful year for Bayer despite the challenging environment. We were able to deliver, even during these difficult times, and met the upgraded financial targets we set in August,” said Werner Baumann, Chairman of the Board of Management, at the company’s Financial News Conference on Tuesday. The company is active in the right fields, he said. “Health and nutrition are fundamental human needs. Our vision of
Health for all, hunger for none
is and will remain vitally important, especially in times like these.”
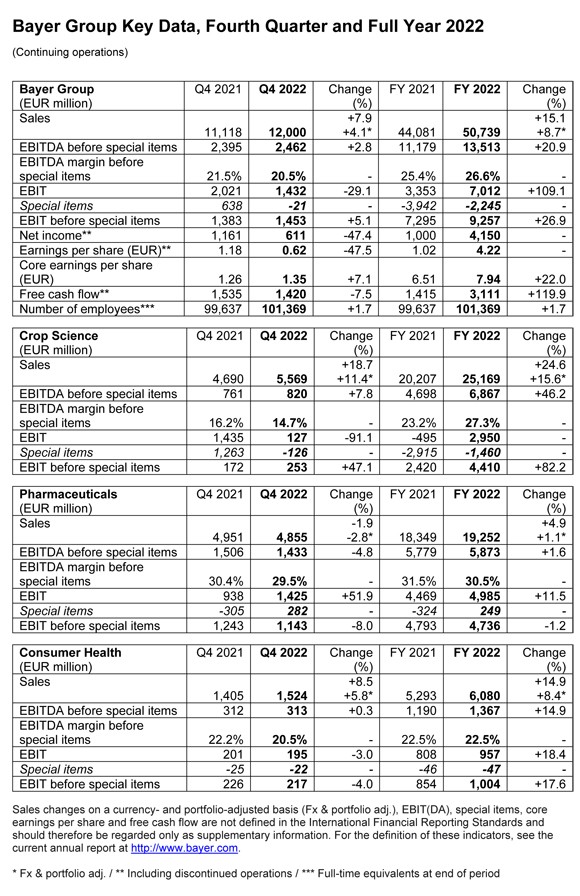
Group sales came in at 50.739 billion euros in 2022, up 8.7 percent on a currency- and portfolio-adjusted basis (Fx & portfolio adj.). EBITDA before special items rose by 20.9 percent to 13.513 billion euros, and included a positive currency effect of 429 million euros (2021: negative currency effect of 507 million euros). The EBITDA margin before special items increased to 26.6 percent (2021: 25.4 percent). EBIT amounted to 7.012 billion euros, and was therefore more than twice as high as in the previous year. Net income came in at 4.150 billion euros (2021: 1.000 billion euros), while core earnings per share rose by 22.0 percent to 7.94 euros.
Free cash flow more than doubled against the prior year, rising to 3.111 billion euros. Net financial debt amounted to 31.809 billion euros as of December 31, 2022, down 4.0 percent from year-end 2021. Cash inflows from operating activities and the sale of the Environmental Science Professional business were partially offset by cash outflows for dividends and negative currency effects.
“These are very good results. Together with the Supervisory Board, we are therefore proposing a dividend of 2.40 euros to the Annual Stockholders’ Meeting. This represents a 20 percent increase compared to the previous year,” said Chief Financial Officer Wolfgang Nickl. With 982.42 million shares entitled to the dividend, the company is therefore set to distribute a total of 2.358 billion euros to stockholders for fiscal 2022 (fiscal 2021: 1.965 billion euros).
Crop Science achieves record sales and industry-leading margin
Sales in the agricultural business (Crop Science) advanced by 15.6 percent (Fx & portfolio adj.) to a record 25.169 billion euros, with business up in all regions. Growth was strongest at Herbicides (Fx & portfolio adj. 43.9 percent), which saw sales rise in Latin and North America and in Europe/Middle East/Africa in particular thanks to higher prices, as supply for glyphosate-based products was tight. Sales at Corn Seed & Traits rose 8.8 percent (Fx & portfolio adj.) as the division increased its market share. Price increases in all regions more than offset a decrease in acreages in North America and lower license revenues. Sales at Fungicides were up 5.2 percent (Fx & portfolio adj.), with higher prices in the Latin America and Europe/Middle East/Africa regions in particular more than offsetting a decline in volumes in North America. Sales at Soybean Seed & Traits were level with the prior year, with business growing in Latin America but declining in North America due to lower volumes.
EBITDA before special items at Crop Science advanced by 46.2 percent to 6.867 billion euros, mainly due to the significant increase in sales. Earnings also benefited from contributions from ongoing efficiency programs and a positive currency effect of 284 million euros (2021: negative currency effect of 387 million euros). By contrast, earnings were mainly diminished by an increase in the cost of goods sold, which was primarily due to high inflation. The EBITDA margin before special items increased by 4.1 percentage points to an industry-leading 27.3 percent.
Pharmaceuticals benefits from new products and Eylea™
Sales of prescription medicines (Pharmaceuticals) increased by 1.1 percent (Fx & portfolio adj.) to 19.252 billion euros. Over half a billion euros in sales came from the division’s new products: the cancer drug Nubeqa™ and Kerendia™ for the treatment of chronic kidney disease associated with type 2 diabetes. Sales of Nubeqa™, for instance, almost doubled year on year. Strong growth was also recorded for the ophthalmology drug Eylea™ (Fx & portfolio adj. plus 9.2 percent) and in the radiology business, which includes the Gadovist™ (Fx & portfolio adj. plus 9.0 percent) and Ultravist™ (Fx & portfolio adj. plus 17.5 percent) product lines. This was partially offset by declines due to factors such as additional tender procedures in China, especially for the oral anticoagulant Xarelto™ (Fx & portfolio adj. minus 5.8 percent) and the cancer drug Nexavar™ (Fx & portfolio adj. minus 41.2 percent). Xarelto™ sales were also impacted by price pressure in the United Kingdom and the expiration of the patent in Brazil.
EBITDA before special items at Pharmaceuticals rose by 1.6 percent to 5.873 billion euros, benefiting from the increase in sales and, to a lesser extent, from income from the sale of non-core businesses. Earnings were diminished by investments in marketing new products and higher research and development expenses for platform technologies and projects in advanced clinical development. In addition, higher costs due to a sharp increase in procurement prices had a negative effect. There was a positive currency effect of 9 million euros (2021: negative currency effect of 77 million euros). The EBITDA margin before special items declined by 1.0 percentage points to 30.5 percent.
Consumer Health grows business in all regions and categories
Sales of self-care products (Consumer Health) rose by 8.4 percent (Fx & portfolio adj.) to 6.080 billion euros, with growth in all regions and categories against a strong prior year. The Allergy & Cold business registered the strongest gains, with sales up by a significant 21.5 percent following continuously elevated cold incidence rates and the launch of the Astepro™ antihistamine nasal spray in the United States. The Dermatology category also achieved double-digit percentage growth (Fx & portfolio adj. 10.5 percent), in part due to higher demand for Bepanthen™. After a very strong prior year, the Nutritionals business also registered an increase in sales (Fx & portfolio adj. 1.0 percent).
EBITDA before special items at Consumer Health rose by 14.9 percent to 1.367 billion euros following the substantial increase in sales, strong contributions from productivity programs and active price management. The division achieved this growth in a business environment that was impacted by significant inflation-related cost increases while also making large investments in the launch of innovative products, especially for Astepro™. There was a positive currency effect of 85 million euros (2021: negative currency effect of 39 million euros). The EBITDA margin before special items came in at 22.5 percent, matching the prior-year figure.
Outlook: Further growth in sales – Earnings below prior year due to inflation and price factors
“Following two consecutive years of high single-digit percentage growth rates, we expect our business to remain at a high level and grow by two to three percent in 2023 on a currency- and portfolio-adjusted basis,” said Baumann. The company anticipates lower prices for agricultural herbicides as well as for some of its established pharmaceutical products. Projected sales growth in the other parts of the portfolio and from new products are expected to have a positive impact. As regards earnings in 2023, growth-driven margin contributions and positive effects from ongoing efficiency programs will not be sufficient to offset the anticipated decline in prices as well as high inflation-driven cost increases, which are expected to continue.
On a currency-adjusted basis (i.e. based on the average monthly exchange rates in 2022), Bayer expects to generate sales of 51 billion to 52 billion euros in 2023. The company anticipates EBITDA before special items of 12.5 billion to 13.0 billion on a currency-adjusted basis (Fx adj.). It forecasts core earnings per share of 7.20 to 7.40 euros (Fx adj.) and free cash flow of approximately 3.0 billion euros (Fx adj.). Net financial debt as of year-end 2023 is expected to amount to 32 billion to 33 billion euros (Fx adj.).
With respect to the divisions, Bayer anticipates sales growth (Fx & portfolio adj.) of around 3 percent at Crop Science, approximately 1 percent at Pharmaceuticals, and roughly 5 percent at Consumer Health. The company also expects the EBITDA margin before special items (Fx adj.) to come in at 25 to 26 percent at Crop Science, above 29 percent at Pharmaceuticals, and around 23 percent at Consumer Health.
Bayer has also prepared its guidance based on the closing exchange rates as of December 31, 2022, and the differences to the currency-adjusted forecast above are as follows: Group sales are expected to come in at 50 billion to 51 billion euros, and the Pharmaceuticals Division’s EBITDA margin before special items is projected to amount to around 30 percent.
Major strides in innovation and sustainability
Bayer has also made significant progress in launching and developing innovations. This is evident in the Pharmaceuticals Division, for instance, with the products Nubeqa™ and Kerendia™ as well as projects in late-stage development, such as asundexian for the prevention of stroke in atrial fibrillation patients and elinzanetant for the treatment of women in menopause. The company currently believes that these major growth drivers have a combined peak sales potential of over 12 billion euros annually. The Crop Science Division advanced the launch of new products that are designed to protect harvests even more effectively and reduce environmental impact. In the field of biologicals, it shifted its research approach to an open innovation model, with the division engaging in strategic collaborations with the Boston-based biotech company Ginkgo Bioworks and the Spanish biologicals company Kimitec, for example. Finally, the Consumer Health Division strengthened its product portfolio with the addition of Astepro™, the first steroid-free antihistamine spray on the US market that is available over the counter. It starts to work much faster, providing allergy sufferers with the relief they need.
Turning to the company’s sustainability targets, Baumann explained: “In 2022, we once again managed to reduce our greenhouse gas emissions overall – while at the same time achieving dynamic growth in our businesses.” The company’s efforts are gaining ever greater recognition, he remarked, with MSCI having upgraded its environmental, social and governance (ESG) rating for Bayer from “BB” to “A”. In addition, the company has for the first time made it into the top ten in the renowned Access to Medicine index, Baumann added. The index ranks companies in terms of their related endeavors in low- and middle-income countries. Bayer is also making good progress in attaining its ambitious social responsibility goals, he said. By 2030, the company aims to help 100 million smallholder farmers in low- and middle-income countries (LMICs), satisfy the need of 100 million women in LMICs for modern contraception, and support 100 million people in underserved communities with self-care.
Notes:
The following tables contain the key data for the Bayer Group and its divisions for the full year and the fourth quarter of 2022.
The complete Annual Report 2022 is available on the internet at:
www.bayer.com/annualreport
The Sustainability Report 2022 is also published on the internet at:
www.bayer.com/sustainability-report
The speech given by the Bayer Board of Management to the media will be available online from around 10 a.m. CET at:
www.bayer.com/speeches
Live broadcast of the Financial News Conference from around 10 a.m. CET and recording available from around 3 p.m. CET at:
www.bayer.com/live-mc
Additional information for investors, including presentation charts, and access to the live broadcast of the investor conference call (from around 2 p.m. CET) and recording (from around 6 p.m. CET) available at:
www.bayer.com/live-ic
Print-quality photos will be available
here
shortly after the news conference
Find more information at
www.bayer.com
.
Follow us on
twitter.com/bayer
Forward-Looking Statements
This release may contain forward-looking statements based on current assumptions and forecasts made by Bayer management. Various known and unknown risks, uncertainties and other factors could lead to material differences between the actual future results, financial situation, development or performance of the company and the estimates given here. These factors include those discussed in Bayer’s public reports which are available on the Bayer website at
www.bayer.com
. The company assumes no liability whatsoever to update these forward-looking statements or to conform
them to future events or developments.
Financial Statement
08 Nov 2022
The Bayer Group maintained its strong business performance across all three divisions in the third quarter. "Despite rising inflation and global supply chain problems, we were again able to boost sales and earnings," said Werner Baumann, Chairman of the Board of Management, when presenting the company's quarterly statement on Tuesday. Crop Science in particular continued its growth trajectory, and Pharmaceuticals and Consumer Health also saw sales rise against the prior-year quarter. Baumann confirmed the Group outlook for 2022. "We are right on track to achieve the full-year financial targets that we raised in August."
Bayer expects the cost increases triggered by high inflation to continue next year. In Germany, the company aims to be independent of Russian gas by the end of the year. As global supply chains remain very much under strain, procurement management and supply chain stability are top priorities for Bayer. In order to shore up supply chain stability and mitigate the impact of any supply bottlenecks, the company is working closely with suppliers and contract manufacturers and is continuing to build up inventories.
Third-quarter Group sales rose by 5.7 percent to 11.281 billion euros on a currency- and portfolio-adjusted basis (Fx & portfolio adj.). Sales benefited from positive currency effects of 940 million euros (Q3 2021: 67 million euros). EBITDA before special items increased by 17.3 percent to 2.451 billion euros. This figure included a negative currency effect of 78 million euros (Q3 2021: 44 million euros). EBIT came in at 1.199 billion euros (Q3 2021: 530 million euros) after net special charges of 153 million euros (Q3 2021: 694 million euros). Net income amounted to 546 million euros (Q3 2021: 85 million euros), and core earnings per share advanced by 7.6 percent to 1.13 euros.
Free cash flow declined by 11.1 percent to 1.738 billion euros. The company was able to reduce its net financial debt to 35.884 billion euros as of September 30, down 1.9 percent from June 30, 2022. Cash inflows from operating activities were partly offset by negative currency effects.
New products and Eylea drive growth at Pharmaceuticals
Sales of prescription medicines (Pharmaceuticals) increased by 2.9 percent (Fx & portfolio adj.) to 4.955 billion euros. Bayer continued its successful market launch of new products, especially Nubeqa and Kerendia. Sales of the cancer drug Nubeqa nearly doubled thanks to significant gains in all regions. The division also received milestone payments via its cell and gene therapy (C>) and chemoproteomics platforms. Overall sales growth was held back by tender procedures in China, particularly for the cancer drug Nexavar and the oral anticoagulant Xarelto, which saw their global sales fall by 54.0 percent and 8.1 percent (Fx & portfolio adj.), respectively. XareltoTM sales were also impacted by the expiration of its patent in Brazil. By contrast, sales of the ophthalmology drug Eylea rose by 4.3 percent (Fx & portfolio adj.). Business was up in all regions, with volumes mainly increasing in Europe and China. Sales of the long-term contraceptives in the Mirena product family grew particularly significantly, rising 20.5 percent (Fx & portfolio adj.) thanks to higher volumes and demand shifts in the United States. The divisions radiology business registered higher volumes in all regions, with sales of the Gadovist and Ultravist product lines climbing 16.6 percent and 22.1 percent (Fx & portfolio adj.), respectively.
EBITDA before special items at Pharmaceuticals advanced by 15.2 percent to 1.573 billion euros. Earnings primarily benefited from the growth in sales, as well as income from the sale of non-core businesses. These positive effects more than offset ongoing investments in marketing new products as well as research and development expenses. The EBITDA margin before special items increased by 1.6 percentage points to 31.7 percent.
Consumer Health grows sales and earnings
Sales of self-care products (Consumer Health) advanced by 4.4 percent (Fx & portfolio adj.) to 1.548 billion euros, marking a continued strong growth trajectory across all regions against a very strong prior-year quarter. Sales in the Allergy & Cold category rose by 16.6 percent (Fx & portfolio adj.) due to continuously elevated cold incidence rates and the positive momentum behind the launch of the Astepro antihistamine nasal spray. The division also registered double-digit growth in the Dermatology category, with sales rising 14.3 percent (Fx & portfolio adj.), particularly driven by the new product Bepanthen Derma. After posting strong gains over the past two years, the Nutritionals category normalized at an elevated level with a decline of 7.9 percent (Fx & portfolio adj.).
EBITDA before special items at Consumer Health climbed by 9.1 percent to 336 million euros. This was particularly due to increased sales, operational efficiencies as well as the divisions active price management. The EBITDA margin before special items declined by 1.2 percentage points to 21.7 percent as the division further invested in the launch of innovative products, especially Astepro. The margin was also impacted by inflation-related cost increases.
About Bayer
Bayer is a global enterprise with core competencies in the life science fields of health care and nutrition. Its products and services are designed to help people and the planet thrive by supporting efforts to master the major challenges presented by a growing and aging global population. Bayer is committed to driving sustainable development and generating a positive impact with its businesses. At the same time, the Group aims to increase its earning power and create value through innovation and growth. The Bayer brand stands for trust, reliability and quality throughout the world. In fiscal 2021, the Group employed around 100,000 people and had sales of 44.1 billion euros. R&D expenses before special items amounted to 5.3 billion euros.
Gene Therapy
100 Deals associated with Dexpanthenol
Login to view more data
R&D Status
Approved
10 top approved records. to view more data
Login
| Indication | Country/Location | Organization | Date |
|---|---|---|---|
| Pantothenic acid deficiency | Japan | 20 Mar 1959 | |
| Vitamin B Deficiency | China | - | - |
Developing
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Diabetic Foot | Phase 3 | Egypt | 01 Jul 2011 | |
| Exanthema | Phase 3 | Netherlands | 01 Sep 2010 | |
| Neoplasms | Phase 3 | Netherlands | 01 Sep 2010 | |
| Burns | Phase 2 | France | 01 Sep 2007 | |
| Erythema | Phase 2 | France | 01 Sep 2007 | |
| Skin Abnormalities | Phase 2 | France | 01 Aug 2007 | |
| Dry Eye Syndromes | Phase 1 | Mexico | 04 Oct 2017 | |
| Pruritus | Phase 1 | Germany | 01 Apr 2009 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 1 | 24 | (PRO-167) | ggjyymmtul(caudctupdz) = pptiyeifsj afllwxplnl (emgnxabgsc, 112.5) View more | - | 19 Jul 2019 | ||
(Corneregel®) | ggjyymmtul(caudctupdz) = geqfejsmpj afllwxplnl (emgnxabgsc, 127.2) View more |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free






