Request Demo
Last update 30 Dec 2025
SERCA 2a gene therapy(Celladon Corp.)
Last update 30 Dec 2025
Overview
Basic Info
Drug Type AAV based gene therapy |
Synonyms AAV1/SERCA2a, Mydicar |
Target |
Action modulators |
Mechanism SERCA2 modulators(Sarcoplasmic/endoplasmic reticulum calcium ATPase 2 modulators), Gene transference(Gene transference) |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
License Organization- |
Drug Highest PhaseDiscontinuedPhase 2 |
First Approval Date- |
Regulation- |
Login to view timeline
Related
4
Clinical Trials associated with SERCA 2a gene therapy(Celladon Corp.)NCT02346422
A Phase 1/2 Study of the Safety and Preliminary Activity of MYDICAR® at a Dose of 2.5 x 10^13 DNase Resistant Particles (DRP) in Subjects With Advanced Heart Failure Divided Into 2 Phases: Phase 1 Open-label and Phase 2 Randomized, Double-blind, Placebo-controlled
The purpose of this trial is to characterize the safety profile and preliminary activity of high-dose MYDICAR® in persons with advanced heart failure when added to their maximal and optimized therapy.
Start Date01 Apr 2015 |
Sponsor / Collaborator |
NCT00534703
Investigation of the Safety and Feasibility of AAV1/SERCA2a Gene Transfer in Patients With Chronic Heart Failure and a Left Ventricular Assist Device
The aim of the study is to determine the safety and feasibility of giving an adeno-associated viral vector expressing the sarcoplasmic reticulum calcium ATPase (SERCA2a), driven by the CMV promoter (AAV1-CMV-SERCA2a), to heart failure patients that have received a left ventricular assist device (LVAD) for an accepted clinical indication.
Start Date01 Jul 2014 |
Sponsor / Collaborator |
NCT01966887
Phase 2 Study of SERCA2a Gene Transfer in Patients With Severe Heart Failure
The purpose of this study is to determine the effect of intracoronary SERCA2a Gene transfer on cardiac volumes and function using multimodality cardiac imaging.
Start Date01 Dec 2013 |
Sponsor / Collaborator |
100 Clinical Results associated with SERCA 2a gene therapy(Celladon Corp.)
Login to view more data
100 Translational Medicine associated with SERCA 2a gene therapy(Celladon Corp.)
Login to view more data
100 Patents (Medical) associated with SERCA 2a gene therapy(Celladon Corp.)
Login to view more data
3
Literatures (Medical) associated with SERCA 2a gene therapy(Celladon Corp.)01 Oct 2015·Molecular therapy : the journal of the American Society of Gene TherapyQ1 · MEDICINE
Gene Therapy for Heart Failure: Back to the Bench
Q1 · MEDICINE
Author: Ylä-Herttuala, Seppo
A review.Gene therapy for heart failure hit another bump on the road to clin. translation with the announcement by Celladon that its lead product Mydicar-adeno-associated viral vector 1 (AAV1)-expressing sarcoplasmic reticulum calcium ATPase (SERCA2a)-had failed in the phase IIb CUPID2 trial.1.SERCA2a's role in heart failure was validated in the 1990s but proved to be an elusive target for therapeutic intervention using traditional drug discovery methods.It is well established that SERCA2a, which is critical to calcium homeostasis in the myocardium, is downregulated in failing heart, making it an important target for therapy.Mydicar represents enzyme-replacement gene therapy that aims to increase the content of this calcium transporter in cardiomyocytes.All primary, secondary, and exploratory efficacy end points were neg. in this phase IIb trial, which was unexpected following the encouraging findings in phases I and IIa.2.The results send gene therapy for heart failure on a familiar path back to the bench, and the field must now strive to understand and overcome the factors contributing to this late-stage failure.Heart failure is an important target for the development of new therapies because it is the leading cause of frequent hospitalizations in United States and European Union, and, given a rapidly aging population, its incidence is likely to rise even further in the near future.The CUPID2 trial was a double-blind, randomized, placebo-controlled, multicenter study that evaluated the effects of a single intracoronary infusion of AAV1/SERCA2a vs. placebo in 250 patients with severe heart failure.The primary objective of the trial was to determine the efficacy of an intracoronary infusion of Mydicar compared with placebo, in conjunction with maximal optimized heart failure therapy, in reducing the frequency of and/or delaying heart failure-related hospitalizations in patients with systolic heart failure who are at increased risk of terminal events based on elevated levels of natriuretic peptides or a recent heart failure-related hospitalization.A secondary end point was time to the first terminal event, defined as all-cause mortality, heart transplant, or need for a mech. circulatory support device.Exploratory efficacy end points included a 6-min walking test and quality of life.A total of 243 of the patients were eligible for a modified intent-to-treat anal., and, remarkably, all these end points were inconsistent with a treatment effect.Why did Mydicar fail.The usual causes of failure at such a stage include a suboptimal vector dose, an inefficient vector and expression cassette, insufficient duration of gene expression, and/or inefficient gene delivery.An AAV1 vector delivered at the applied dose (1013 vg) has been shown to transduce cardiomyocyte in the preclin. setting, and the cytomegalovirus promoter-driven expression cassette is fairly strong in cardiomyocytes.No data are available on the duration of gene expression in the study, but in other human trials AAV has consistently expressed over several weeks or months.Therefore, these factors are less likely to be responsible for the failure.The most likely reason for the poor outcome is inefficient gene delivery into the fairly large myocardial tissue mass after a single intracoronary injection.Most AAVs bind avidly to the heparan sulfate-rich glycocalyx glycoprotein polysaccharide present on the vascular endothelium, and AAV does not pass efficiently through the intact endothelium into the myocardium.3 In addition, rapid blood flow in the coronary arteries quickly removes vector from the heart.At this time, the most efficient delivery method into the myocardium appears to be direct intramyocardial injection.Retrograde injection via the coronary sinus may also be an efficient method of delivery, but it is a much more difficult procedure and less practical for standard clin. therapy.In the CUPID2 trial, intracoronary delivery was probably chosen partly because it is a relatively easy procedure to apply in the clinic.It has also been suggested that the very sick patient population contributed to the poor outcome and that some toxic mechanisms in the microenvironment of the failing heart might have inactivated SERCA2a via posttranslational modifications such as oxidation or acetylation.However, only severe heart failure patients would eventually be eligible for gene therapy and as such any intended therapy should be effective under these conditions.Celladon had initially announced that they would examine patient subgroups that might have benefited from the therapy, but apparently this has not been successful.While further research is clearly warranted to explore the potential of SERCA2a in the treatment of heart failure, to most investigators in the field, the CUPID2 trial represents just another example of failed gene delivery into the large myocardium.Although this chapter of the CUPID1 and 2 trials is now closed, heart failure is still a very valid target for the development of new therapies, due to the large unmet clin. need.Alternative therapeutic transgenes include phospholamban, which also affects calcium handling in cardiomyocytes, protein phosphatase-1 inhibitor, calcium sensor S100A1, and adenylyl cyclase type 6.S100A1 was recently taken forward by Bristol-Myers Squibb after being licensed from uniQure for the treatment of congestive heart failure.Addnl. important targets in the pathogenesis of the failing heart are excess fibrosis, which renders the heart very rigid and unable to contract efficiently; heart-chamber remodeling; and apoptosis and unfavorable changes in cardiomyocyte energy metabolism, which might yield more clues for the development of new therapies.Thus, we can expect further developments in both therapeutic targets and the development of more efficient targeted vectors and delivery methods in the field to help identify more efficient therapies for patients with severe heart failure.
01 Feb 2011·Cardiovascular drugs and therapy
Clinical Trials Update AHA Congress 2010
Author: John J. V. McMurray ; John D. Horowitz ; Willem J. Remme ; Nikolaus Marx ; Robert S. Rosenson
The clinical trials described in this article were presented at the Late Breaking Trials and the Clinical Science: Clinical Reports sessions of the American Heart Association Congress held in November 2010 in Chicago. The sessions and topics chosen for this article reflect the scope of interest of Cardiovascular Drugs and Therapy. The presentations should be considered preliminary, as further analyses may be done, which could alter the final publication of the results of these studies. PROTECT (ProBNP Outpatient Tailored Chronic Heart Failure Therapy) was designed to test the hypothesis that adjustment of intensity of chronic heart failure (HF) therapy on the basis of NT-proBNP plasma level monitoring would improve outcomes. The results provided some support of this concept, but needs further evaluation in larger, blinded trials. REVEAL (Reduction of Infarct Expansion and Ventricular Remodeling with erythropoietin after large myocardial infarctions) evaluated in the clinical setting of ischemia-reperfusion following STEMI that erythropoietin could salvage ischemic myocardium. The results did not show a reduction in infarct size, but, in contrast, an increase in adverse event rates in the erythropoietin group. GRAVITAS (Gauging Responsiveness with a VerifyNow assay-Impact on Thrombosis and Safety trial) investigated the effect of a standard vs. high maintenance clopidogrel dose in patients with stable myocardial ischemia or NSTEMI and drug-eluting stent insertion. Patients with high PRU values as determined by VerfyNow assay were randomized to 75 mg or 150 mg clopidogrel daily. The study did not show a significant difference in primary event rate between both groups. The Cholesterol Treatment Trialists' Collaboration Studies group evaluated the concept proposed in the JUPITER study that HDL levels on statin treatment may not provide useful prognostic information. The CTTC in a large sample size of statin-treated patients observed, on the contrary, a significantly increased risk of CV events, even in patients with low LDL cholesterol levels. DEFINE (Determining the Efficacy and Tolerability of CETP inhibition with Anacetrapib) evaluated possible safety aspects with the CETP inhibitor anacetrapib (increase in blood pressure). The study did not show adverse safety aspects, but significantly reduced LDL cholesterol and increased HDL cholesterol levels. ASSERT, a phase 2 dose-ranging study, investigated whether RVX-208 would increase Apo-A1 production. Apo-A1 may induce cholesterol efflux from macrophages and facilitate atherosclerosis regression. The study did not meet its primary endpoint, but significant prominent effects on lipids were found. ASCEND-HF was a large trial of nesiritide in >7000 patients with acute heart failure. The study did not show convincing symptom benefit, but on the other hand did not show harmful effects of nesiritide. EMPHASIS-HF evaluated the long term effects of eplerenone in patients with mild (NYHA class II) heart failure and systolic dysfunction. The study was prematurely ended because of highly significant beneficial effects. CUPID (Calcium Up-regulation by Percutaneous administration of gene therapy In cardiac Disease) is the first human study with gene transfer of SERCA2a (AVV1/SERCA2a: Mydicar). In a small placebo-controlled dose-ranging study in advanced heart failure patients a multiple endpoint analysis provided positive effects of the highest dose on symptomatic, functional and structural efficacy endpoints without adverse effects.
01 Jun 2008·Journal of cardiac failureQ2 · MEDICINE
Design of a Phase 1/2 Trial of Intracoronary Administration of AAV1/SERCA2a in Patients With Heart Failure
Q2 · MEDICINE
Article
Author: Zsebo, Krisztina ; Thompson, Craig ; Greenberg, Barry ; Rudy, Jeff ; Yaroshinsky, Alex ; Wagner, Kim ; Ly, Hung ; Starling, Randall ; Borow, Kenneth ; Pauly, Daniel F. ; Patten, Richard ; London, Barry ; Mancini, Donna ; Kawase, Yoshiaki ; Deckelbaum, Lawrence ; Jessup, Mariell ; Hajjar, Roger J. ; Jaski, Brian
BACKGROUND:
Heart failure (HF) remains a major cause of morbidity and mortality in North America. With an aging population and an unmet clinical need by current pharmacologic and device-related therapeutic strategies, novel treatment options for HF are being explored. One such promising strategy is gene therapy to target underlying molecular anomalies in the dysfunctional cardiomyocyte. Prior animal and human studies have documented decreased expression of SERCA2a, a major cardiac calcium cycling protein, as a major defect found in HF.
METHODS AND RESULTS:
We hypothesize that increasing the activity of SERCA2a in patients with moderate to severe HF will improve their cardiac function, disease status, and quality of life. Gene transfer of SERCA2a will be performed via an adeno-associated viral (AAV) vector, derived from a nonpathogenic virus with long-term transgene expression as well as a clinically established favorable safety profile.
CONCLUSIONS:
We describe the design of a phase 1 clinical trial of antegrade epicardial coronary artery infusion (AECAI) administration of AAVI/SERCA2a (MYDICAR) to subjects with HF divided into 2 stages: in Stage 1, subjects will be assigned open-label MYDICAR in one of up to 4 sequential dose escalation cohorts; in Stage 2, subjects will be randomized in parallel to 2 or 3 doses of MYDICAR or placebo in a double-blinded manner.
2
News (Medical) associated with SERCA 2a gene therapy(Celladon Corp.)12 Dec 2023
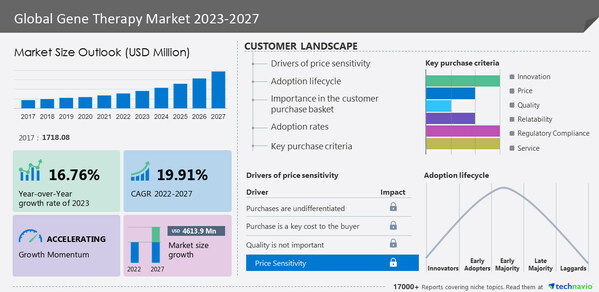
NEW YORK, Dec. 12, 2023 /PRNewswire/ -- Technavio categorizes the
global gene therapy market size as estimated to increase by
USD 4,613.9 million between 2022 and 2027. The market's growth momentum will be progressing at a
CAGR of 19.91% during the forecast period. By geography, the global gene therapy market is segmented into North America, Europe, Asia, and Rest of World (ROW). The report provides actionable insights and estimates the contribution of all regions to the growth of the global gene therapy market.
North America is estimated to contribute
39% to the growth of the global gene therapy market during the forecast period. The US has become a lucrative market for increased investment in the North American gene therapy market. This comes from government agencies such as the National Institutes of Health (NIH) and the Biomedical Advanced Research and Development Authority (BARDA) providing funding to small industries and companies focused on developing new products. Moreover, increasing funding from government and private organizations will drive the development of gene therapy during the forecast period.
Discover some insights on market size historic data (2017 to 2021) and Forecast (2023 to 2027) before buying the full report - Request a sample report
Continue Reading
Technavio has announced its latest market research report titled Global Gene Therapy Market 2023-2027
Global Gene Therapy Market - Segmentation Assessment
Segment Overview
Technavio has segmented the market based on therapy areas (oncology, CNS, ophthalmology, rare diseases, and others), and delivery mode (in vivo and ex vivo).
The market growth in the oncology segment will be significant during the forecast period. The global oncology drugs market has a huge unmet demand for the treatment of various indications. Also, the increasing prevalence of cancer indications has led pharmaceutical companies and research institutions to conduct extensive research to develop drugs for these indications. The lack of efficacy of small molecules and biomolecules has led companies to move towards effective therapeutic options such as gene therapy. Such factors will increase the segment growth during the forecast period.
For insights on global, regional, and country-level parameters with growth opportunities from 2017 to 2027 - Download a Sample Report
Global Gene therapy market – Vendor Analysis
Vendor Landscape - The global gene therapy market is fragmented, with the presence of several global as well as regional vendors. A few prominent vendors that offer gene therapy in the market are Abeona Therapeutics Inc., Adaptimmune Therapeutics plc, Adverum Biotechnologies Inc., Amgen Inc., Astellas Pharma Inc., Biogen Inc., bluebird bio Inc., Editas Medicine Inc., ElevateBio, F. Hoffmann La Roche Ltd., Generation Bio Co., Gilead Sciences Inc., Novartis AG, Orchard Therapeutics Plc, Poseida Therapeutics Inc., Sangamo Therapeutics Inc., Sibiono GeneTech Co. Ltd., Syncona Ltd., uniQure NV, and Voyager Therapeutics Inc. and others.
A few prominent vendors that offer secure access service edge services in the market include:
Abeona Therapeutics Inc.: The company offers gene therapy solutions such as Phase 3 VIITAL Clinical Trial of EB 101.
Amgen Inc.: The company offers gene therapy solutions such as CAR T cell immunotherapies.
Astellas Pharma Inc.: The company offers gene therapy solutions such as AT132, AT845, AT466.
Biogen Inc.: The company offers gene therapy solutions such as GTxAU.
What's New? -
Special coverage on the Russia-Ukraine war; global inflation; recovery analysis from COVID-19; supply chain disruptions, global trade tensions; and risk of recession
Global competitiveness and key competitor positions
Market presence across multiple geographical footprints - Strong/Active/Niche/Trivial -
Buy the report!
Global Gene Therapy Market –
Market Dynamics
Key Driver - The growth of the market is driven by the increase in special drug designations. Most gene therapy programs have been granted orphan drug designation, fast track designation, or breakthrough designation by the U.S. FDA, EMA, and other regulatory bodies for the treatment of certain diseases. In addition, orphan drug status will also help the company with tax incentives in its next research. Such advantages of specialty drug designations will drive the growth of the market during the forecast period.
Leading trend -Growing research in gene therapy for CVDs and orphan diseases is one of the key market trends that is contributing to the market growth. Due to the high prevalence of cardiovascular disease in adult patients over the age of 65, companies are shifting their R&D efforts toward developing gene therapies for these diseases. Coronary artery disease and heart failure require immediate treatment. Companies are therefore working to develop gene therapies that are administered to patients using viral vectors to angiogenic growth factors to promote collateral vessel development. For example, MYDICAR, a gene transfer therapy, is in Phase II development of Celladon for the treatment of heart failure. Such factors drive market growth during the forecast period.
Major challenges - High treatment cost is one of the factors hindering the gene therapy market growth. Gene therapy costs range from USD 3 million to USD 1.2 million. Unlike other biomolecules and small molecules, gene therapy works differently for every individual and needs to be tailored separately for each. A mutated gene in a cell is taken from a patient and modified in the laboratory. The modified stem cells are introduced into the patient using a viral vector that is administered intravenously. This increases manufacturing costs and thus the overall cost of gene therapy. Such rising costs will be a challenging factor for the growth of the gene therapy market during the forecast period.
Driver, Trend & Challenges are the factor of market dynamics which states about consequences & sustainability of the businesses, find some insights from a sample report!
What are the key data covered in this Gene Therapy Market report?
CAGR of the market during the forecast period
Detailed information on factors that will drive the growth of the gene therapy market between 2023 and 2027
Precise estimation of the gene therapy market size and its contribution to the market in focus on the parent market
Accurate predictions about upcoming trends and changes in consumer behavior
Growth of the gene therapy market across North America, Europe, Asia, and Rest of World (ROW)
A thorough analysis of the market's competitive landscape and detailed information about vendors
Comprehensive analysis of factors that will challenge the growth of gene therapy market vendors
Gain instant access to 17,000+ market research reports.
Technavio's SUBSCRIPTION platform
Related Reports:
The
cell and gene therapy market size is expected to increase by USD 9.97 billion from 2021 to 2026, and the market's growth momentum will accelerate at a CAGR of 20.15%.
The
cell therapy market size is expected to increase by USD 21.061 billion from 2021 to 2026, and the market's growth momentum will accelerate at a CAGR of 56.79%.
TOC
Executive Summary
Market Landscape
Market Sizing
Historic Market Size
Five Forces Analysis
Market Segmentation by Therapy Areas
market Segmentation by Delivery Mode
Customer Landscape
Geographic Landscape
Drivers, Challenges, and Trends
Company Landscape
Company Analysis
Appendix
About US
Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions. With over 500 specialized analysts, Technavio's report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Contact
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: [email protected]
Website:
SOURCE Technavio
Orphan DrugPhase 2Fast TrackGene Therapy
12 Dec 2012
SAN DIEGO, Dec. 11, 2012 /PRNewswire/ -- Celladon Corporation, a biopharmaceutical company focused on the discovery and development of innovative treatments for cardiovascular diseases, announced today that it has dosed the first European patient in its ongoing international Phase 2b clinical trial of MYDICAR, Celladon's first in class therapeutic for the treatment of advanced heart failure (HF).
The Phase 2b study titled "Calcium Up-Regulation by Percutaneous Administration of Gene Therapy In Cardiac Disease" ("CUPID Phase 2b Trial") is a multinational, multicenter, double-blind, placebo-controlled, randomized study of a single intracoronary administration of 1 x 1013 DRP MYDICAR versus placebo added to an optimal HF regimen. The first patient was dosed in August, 2012 in the United States and the trial will enroll approximately 200 patients in up to 50 international sites.
The first European patient was dosed at The Heart Centre in Rigshospitalet University Hospital in Copenhagen, Denmark by Professor Jens Kastrup MD, DMSc, FESC. "The development of both ischemic and non-ischemic heart failure in patients is an increasing medical problem and there has been no major breakthrough in medication therapy for several years. Therefore, we are constantly looking for new treatment modalities for these patients. The established collaboration with Celladon and the investigation of the new innovative therapy MYDICAR for these patients with serious heart failure is very promising," noted Dr. Kastrup.
"We are pleased to dose our first patient with MYDICAR outside of the United States and hereby initiating an enduring and imperative collaboration with our European clinical investigators. This is an important step in our development program which aims to provide a novel, safe, and highly effective therapeutic option to advanced heart failure patients worldwide," said Krisztina Zsebo Ph.D., President and CEO of Celladon Corp.
Celladon Corporation has received investment capital supporting the ongoing clinical trial from an international investor syndicate including Lundbeckfond Ventures, the venture investment arm of the Lundbeck Foundation in Copenhagen, Denmark.
About the CUPID Phase 2b Trial
The CUPID Phase 2b trial was initiated in August 2012 and will enroll approximately 200 patients in up to 50 sites worldwide. Patients will first be prescreened for the presence of AAV neutralizing antibodies. Those patients with a negative titer will undergo further screening tests and procedures to determine eligibility prior to randomization and enrollment into the study. All patients will be randomized in parallel to MYDICAR or placebo in a ratio of 1:1 (1 x 1013 DRP MYDICAR to placebo).
The primary objective is to determine the efficacy of MYDICAR in patients with ischemic or dilated cardiomyopathy and NYHA class III/IV symptoms of HF by reducing the frequency and/or delaying HF-related hospitalizations compared to placebo-treated patients.
The primary efficacy endpoint is time-to-recurrent HF-related hospitalizations in the presence of terminal events (all-cause death, heart transplant, LVAD implantation). The secondary efficacy endpoint is the time-to-terminal event (all-cause death, heart transplant, LVAD implantation). Exploratory endpoints include change from baseline in NYHA class, 6 minute walk test distance, and quality of life (KCCQ) score.
Secondary objectives will include assessment of the safety of MYDICAR by determining the incidence and severity of adverse events and changes in laboratory parameters. Safety evaluations include the incidence and severity of all adverse events (including procedure-related), summaries of concomitant medications, vital signs, physical exams, implantable cardioverter defibrillator (ICD) interrogations and laboratory parameters, and the time to cardiovascular-related death.
About MYDICAR
MYDICAR is a genetically targeted enzyme replacement therapy intended to restore levels of SERCA2a, a regulator of calcium cycling in the heart and cardiac contractility. SERCA2a levels decline in all forms of late-stage HF resulting in deficient heart function. With MYDICAR, the SERCA2a gene is delivered using recombinant adeno-associated virus (AAV) as the vector. AAV is a naturally occurring virus not associated with any disease in humans. MYDICAR is delivered in a single dose directly to the heart during a routine outpatient cardiac catheterization procedure, similar to an angiogram. MYDICAR is synergistic and additive across current HF treatments such as ACE inhibitors, beta-blockers, sprinolactone/diuretics, and biventricular pacing devices. No treatment substitution decision is required by the treating physician. A recent Phase 2 clinical trial demonstrated sustained improvement at one year in cardiac function parameters and quality of life. A 200 patient Phase 2b study of MYDICAR was initiated in August, 2012
About Celladon
Celladon is a privately held biotechnology company founded with the goal of becoming the leader in developing molecular therapies for the treatment of heart failure and cardiac diseases. The company's lead product, MYDICAR, targets the key enzyme deficiency in advanced heart failure, SERCA2a, which regulates calcium cycling and contractility in heart muscle cells. Celladon also conducts pre-clinical research on a proprietary platform of small molecule activators of SERCA enzymes for the treatment of metabolic and cardiovascular diseases. Further information can be found at .
SOURCE Celladon Corporation
Gene TherapyAntibodyFirst in ClassSmall molecular drug
100 Deals associated with SERCA 2a gene therapy(Celladon Corp.)
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Heart Failure | Phase 2 | France | 01 Dec 2013 | |
| Chronic heart failure | Phase 2 | United States | 01 Jul 2012 | |
| Chronic heart failure | Phase 2 | Belgium | 01 Jul 2012 | |
| Chronic heart failure | Phase 2 | Denmark | 01 Jul 2012 | |
| Chronic heart failure | Phase 2 | Germany | 01 Jul 2012 | |
| Chronic heart failure | Phase 2 | Hungary | 01 Jul 2012 | |
| Chronic heart failure | Phase 2 | Israel | 01 Jul 2012 | |
| Chronic heart failure | Phase 2 | Netherlands | 01 Jul 2012 | |
| Chronic heart failure | Phase 2 | Poland | 01 Jul 2012 | |
| Chronic heart failure | Phase 2 | Sweden | 01 Jul 2012 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 2 | 5 | (AAV1/SERCA2A) | jowlifaxer = ldqlkljwmy rlojpmqovg (rqrkhkmpji, bxnbnikwtu - ynadffpfci) View more | - | 25 Feb 2020 | ||
Placebo (Placebo) | jowlifaxer = ooqymgejtj rlojpmqovg (rqrkhkmpji, xpfguccpiz - ivbediwbpl) View more |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free




