Request Demo
Last update 01 Jun 2025
VZV vaccine(Merck Sharp & Dohme Corp.)
Last update 01 Jun 2025
Overview
Basic Info
Drug Type Prophylactic vaccine |
Synonyms Inactivated varicella-zoster vaccine, Varicella zoster virus vaccine, VZV vaccine + [3] |
Target- |
Action stimulants |
Mechanism Immunostimulants |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
License Organization- |
Drug Highest PhaseDiscontinuedPhase 3 |
First Approval Date- |
Regulation- |
Login to view timeline
Related
10
Clinical Trials associated with VZV vaccine(Merck Sharp & Dohme Corp.)CTIS2024-516008-41-00
- VMX-SPN-212-001
Start Date13 Feb 2025 |
Sponsor / Collaborator |
NCT02180295
A Phase III, Double-Blind, Lot-To-Lot Consistency Clinical Trial to Evaluate the Safety, Tolerability and Immunogenicity of V212 in Healthy Adults
The study will evaluate the consistency of 3 lots of inactivated VZV vaccine for safety, tolerability, and immunogenicity in healthy adults. The primary hypothesis of the study is that the 3 lots of inactivated vaccine will demonstrate similar immunogenicity at 28 days after the fourth dose.
Start Date01 Jul 2014 |
Sponsor / Collaborator |
NCT01527383
A Phase II Randomized, Placebo-Controlled Clinical Trial to Study the Safety and Immunogenicity of V212 in Adult Patients With Autoimmune Disease
This is a study to evaluate the safety and immunogenicity of V212 vaccine in adults with autoimmune disease, including participants with rheumatoid arthritis, psoriatic arthritis, psoriasis, inflammatory bowel disease, systemic lupus erythematosus, multiple sclerosis, and other similar diseases. The primary hypothesis is that vaccination with V212 vaccine will elicit significant VZV-specific immune responses at approximately 28 days after vaccination 4. The statistical criterion for significance requires that the lower bound of the 2-sided 95% confidence interval of the geometric mean fold rise in vaccine recipients is >1.0.
Start Date21 Feb 2012 |
Sponsor / Collaborator |
100 Clinical Results associated with VZV vaccine(Merck Sharp & Dohme Corp.)
Login to view more data
100 Translational Medicine associated with VZV vaccine(Merck Sharp & Dohme Corp.)
Login to view more data
100 Patents (Medical) associated with VZV vaccine(Merck Sharp & Dohme Corp.)
Login to view more data
157
Literatures (Medical) associated with VZV vaccine(Merck Sharp & Dohme Corp.)31 Dec 2025·Human Vaccines & Immunotherapeutics
Varicella zoster virus mRNA vaccine candidate induced superior cellular immunity and comparable humoral and Fc-mediated immunity compared to the licensed subunit vaccine in a mouse model
Article
Author: Park, Hosun ; Kim, Yunhwa ; Choi, Seok-Tae ; Nam, Jae-Hwan ; Kim, Keunea ; Jang, Eun-Jeong ; Hwang, Ji-Young ; Seo, Ho Seong ; Kwak, Hye Won ; Woo, Chang-Hoon ; Lee, Kyung-Min ; Lim, Jae Hyang ; Xayaheuang, Sivilay ; Yoon, Boomi
The threat of herpes zoster (HZ) is increasing, particularly in the elderly and immunocompromised individuals. Although two platform vaccines are currently available for HZ prevention, the low effectiveness of the live attenuated varicella-zoster virus vaccine (Zostavax®), and the high reactogenicity and limited supply of the AS01 adjuvant gE subunit vaccine (Shingrix®) indicate that, the development of more effective and safe vaccines is required. Compared to conventional vaccines, mRNA vaccines offer the advantages of faster production and generally do not require adjuvants. However, no authorized mRNA vaccine is currently available for HZ. Therefore, we aimed to prepare a gE mRNA vaccine and evaluate the immunogenicity compared with the two commercial vaccines in mice. The gE mRNA vaccine elicited a robust humoral immune response, as measured by an enzyme-linked immunosorbent assay and the fluorescent antibody to membrane antigen test. The mRNA vaccine binding antibody level was comparable to that of Shingrix® and significantly higher than that of Zostavax®. In contrast, in cellular immune responses, which were evaluated by ELISpot assays and intracellular cytokine staining assay, the VZV gE mRNA vaccine induced significantly higher responses than Zostavax® and Shingrix®. In addition, the antibody-dependent cellular phagocytosis activity of the gE mRNA vaccine was comparable to that of the commercial vaccines. However, the highest antibody-dependent cellular cytotoxicity response was achieved by Shingrix®, followed by gE mRNA and then Zostavax®. Our results demonstrate that the mRNA HZ vaccine candidate elicited robust immunogenicity, especially in cellular immunity, and shows a promising potential for HZ prevention.
01 Apr 2025·VACCINE
Considering recombinant herpes zoster vaccine for fragile pediatric patients: A new opportunity
Article
Author: Mollo, A ; Venturini, E ; Marrani, E ; Lodi, L ; Ricci, S ; Sardi, I ; Tintori, V ; Tondo, A ; Azzari, C ; Astorino, V ; Gissi, A ; Indolfi, G ; Lionetti, P ; Peri, M ; Trapani, S ; Mastrolia, M V ; Galli, L
BACKGROUND:
A recombinant vaccine is approved to prevent herpes zoster (HZ) in adults ≥50 years and immunocompromised individuals ≥19 years. However, in children, the live attenuated vaccine remains the only prevention strategy against varicella zoster virus (VZV), with only one trial evaluating the safety and immunogenicity of GlaxoSmithKline's HZ subunit candidate vaccine in immunocompromised children.
OBJECTIVES:
To estimate VZV burden in our third level pediatric hospital and identify high-risk pediatric groups for its occurrence and complications to explore the need for an inactivated vaccine.
METHODS:
We reviewed VZV/HZ hospital discharge codes and positive VZV molecular tests at Meyer Children's Hospital from January 2018 to May 2023. We categorized patients based on their vaccination status as unvaccinated, partially vaccinated (single dose), or fully vaccinated (complete two-dose regimen). 96 controls from the same Departments and period were also included to assess VZV vaccine effectiveness.
RESULTS:
Of 48 patients with VZV (52 % female; median age: 11.6 years [IQR: 7-14.2]), 10 had chickenpox and 38 HZ; 2/48 (4.2 %) received 2 doses of vaccination, 10/48 (20.8 %) were immunized with 1 dose and 36/48 (75 %) were unvaccinated. Immune-related comorbidities were present in 20/48 (42 %) patients, and among those with HZ requiring hospitalization, comorbidities strongly predicted admission (OR 4.71; 95 % CI, 1.23-20.39; p = 0.028). Full vaccination was more frequent in controls (43/96, 45 %) than in cases (2/48, 4.2 %; p < 0.001).
CONCLUSIONS:
In our cohort, many cases had comorbidities contraindicating the live attenuated vaccine. If proven safe and effective, the recombinant HZ vaccine could offer a preventive option for immunocompromised children ineligible for live viral vaccines.
31 Dec 2024·Emerging Microbes & Infections
Rational optimization of glycoprotein E (gE)-encoding mRNA for improved Varicella-zoster virus mRNA vaccine development
Article
Author: Lin, Ang ; Yang, Chen ; Zhang, Shun ; Zhao, Tongyi ; Di, Zhenhua ; Bu, Lingling ; Cai, Ting ; Zhang, Yujie ; Yang, Yong ; Huang, Lulu
mRNA platform holds promise for next-generation Varicella-zoster Virus (VZV) vaccine development due to its high potency at inducing strong T-cell response. Built upon the design of our 1st-generation VZV mRNA vaccine that encodes for full-length gE antigen, in this study we reported on a novel combinatorial strategy to further optimize the gE-encoding mRNA sequence through signal peptide replacement, C-terminal modification, and insertion of mRNA-stabilizing motif, which collectively contributed to significantly improved vaccine immunogenicity. In adult mice, aged mice, and immunocompromised mice, this optimized VZV mRNA vaccine showed strong superiority in multiple aspects including the induction of gE-specific antibodies, specific memory B-cell response, as well as Th1-type T-cell response.
2
News (Medical) associated with VZV vaccine(Merck Sharp & Dohme Corp.)02 May 2024
Reports first quarter revenues of $167 million, GAAP net loss of $1.2 billion and GAAP diluted EPS of $(3.07)
Prepares for launches of RSV vaccine and Spikevax® 2024-2025 formula; reaffirms 2024 expected product sales of approximately $4 billion
Initiated three new clinical studies evaluating Moderna's investigational individualized neoantigen therapy in combination with Merck's Keytruda® for treatment of patients with bladder cancer, kidney cancer and cutaneous squamous cell carcinoma
Advanced three new vaccine programs (Epstein-Barr virus, Varicella-Zoster virus, norovirus) toward Phase 3 clinical trials as announced at Vaccines Day investor event
CAMBRIDGE, MA / ACCESSWIRE / May 2, 2024 / Moderna, Inc. (NASDAQ:MRNA) today reported financial results and provided business updates for the first quarter of 2024.
"As we anticipate the launches of our Spikevax 2024-2025 formula and RSV vaccine, we are exercising financial discipline and have intensified our focus on building and utilizing AI technologies to further streamline operations and enhance productivity," said Stéphane Bancel, Chief Executive Officer of Moderna. "With 10 late-stage programs, and additional new programs advancing toward pivotal studies, we continue to expect numerous product milestones this year across our vaccines and therapeutics portfolio. This is the start of a banner year for our vaccine platform as we continue to advance mRNA medicines for patients. This is just the beginning."
Recent progress includes:
Commercial Updates
COVID-19: The Company reported $167 million in Spikevax (COVID-19 vaccine) sales in the first quarter of 2024, which includes $100 million of U.S. sales and $67 million of international sales.
In the U.S., the Company is reaffirming its 2024 product sales outlook as it enters the second year of the commercial endemic COVID market. Moderna's focus is on working with public health officials, health care providers and pharmacies to increase vaccination coverage rates to reduce the substantial burden of COVID-19. Moderna is also working with the U.S. FDA and regulators to align the timing of flu and COVID-19 vaccine approvals, which is expected to result in higher vaccination uptake if vaccines are available sooner and offered at the same time as the flu shot.
In the European Union, Moderna is participating in the EU Health Emergency and Response Authority's tendering procedure for up to 36 million doses of mRNA COVID-19 vaccines per year for up to four years.
In the Rest of World, the Company is prioritizing markets for greater commercial focus and in April announced a contract with the Ministry of Health in Brazil (Ministério da Saúde) to supply 12.5 million mRNA COVID-19 vaccines as an integral part of Brazil's 2024 national vaccination campaign against COVID-19.
RSV: The Company anticipates initial regulatory approvals of its RSV vaccine (mRNA-1345) beginning in the first half of 2024.
Moderna is targeting fall 2024 for its U.S. RSV vaccine launch, which will build upon the success of its commercial efforts in the fall COVID-19 market. The Company is encouraged by early indications of widespread consumer awareness and established demand in the RSV market, which Moderna will enter with a strong competitive pro robust clinical efficacy data, a well-established safety and tolerability pro its mRNA technology, and as the only pre-filled syringe (PFS) product available.
The PFS ready-to-use formulation will save pharmacists and clinicians time, potentially alleviating wait times and reducing the burden on pharmacy staff. In a study funded by Moderna, the PFS presentation was three to four times more efficient than vaccines requiring reconstitution, measured in doses per hour and based on mean time of preparation.
First Quarter 2024 Financial Results
Revenue: Total revenue for the first quarter of 2024 was $167 million, compared to $1.9 billion in the same period in 2023. The decline was primarily due to reduced sales of the Company's COVID-19 vaccine. Net product sales for the first quarter of 2024 were $167 million, representing a 91% decline compared to the same period in 2023. This decline aligns with the anticipated transition to a seasonal COVID-19 vaccine market; in the prior year period, the Company recognized revenue primarily from delivered doses deferred from 2022.
Cost of Sales: Cost of sales for the first quarter of 2024 totaled $96 million, which included third-party royalties of $8 million, inventory write-downs of $30 million, and unutilized manufacturing capacity and winddown costs of $27 million. Cost of sales for the first quarter of 2024 decreased by $696 million, or 88%, compared to the same period in 2023. Cost of sales as a percentage of net product sales was 58% for the first quarter of 2024, compared to 43% for the first quarter of 2023. The decrease in cost of sales in 2024 was primarily driven by lower sales volume coupled with reduced unutilized manufacturing capacity, inventory write-downs and losses on firm purchase commitments and related cancellation fees. Conversely, the increase in cost of sales as a percentage of net product sales in 2024 was driven by the lower sales level compared to the prior year.
Research and Development Expenses: Research and development expenses for the first quarter of 2024 decreased by 6% to $1.1 billion, compared to the same quarter of 2023. This reduction was primarily due to the absence of upfront collaboration payments being made in 2024. The upfront payments made in the first quarter of 2023 were related to the Company's strategic collaborations with Generation Bio and Life Edit Therapeutics. Additionally, there was a decrease in clinical development and manufacturing expenses, driven by lower spending on clinical trials for the Company's COVID-19, RSV and seasonal flu programs, which aligns with its planned trial schedules. These decreases were partially offset by higher personnel-related costs, resulting from an increased headcount in the research and development functions.
Selling, General and Administrative Expenses: Selling, general and administrative expenses for the first quarter of 2024 decreased by 10% to $274 million, compared to the first quarter of 2023. This decrease is a result of cost discipline and the efficiencies resulting from investments the Company made in foundational capabilities over the last year, which allowed for a significant reduction of purchased services and the use of external consultants. The Company continues to invest in digital and commercial capabilities and has intensified its focus on building and utilizing AI technologies to further streamline operations and enhance productivity.
Income Taxes: Income tax expense for the first quarter of 2024 was $10 million, in contrast to an income tax benefit of $384 million in the same period last year. The shift primarily resulted from the continued application of a valuation allowance on the majority of the Company's deferred tax assets, first established in the third quarter of 2023. The Company only maintained a valuation allowance on certain state deferred tax assets prior to the third quarter of 2023.
Net Income (Loss): Net loss was $(1.2) billion for the first quarter of 2024, compared to net income of $79 million for the first quarter of 2023.
Earnings (Loss) Per Share: Diluted loss per share was $(3.07) for the first quarter of 2024, compared to diluted earnings per share of $0.19 for the first quarter of 2023.
Cash Position: Cash, cash equivalents and investments as of March 31, 2024, were $12.2 billion, compared to $13.3 billion as of December 31, 2023. The decrease in the Company's cash position during the first quarter of 2024 was largely attributable to research and development expenses and operating activities.
2024 Financial Framework
Expectations for full year 2024 remain consistent with prior expectations.
Revenue: The Company reaffirms its 2024 expected revenue of approximately $4 billion from its respiratory franchise, and now expects approximately $0.3 billion in net sales in the first half of the year, reflecting the seasonality of the respiratory business.
Cost of Sales: Cost of sales is expected to be approximately 35% of product sales for the year.
Research and Development Expenses: Full-year 2024 research and development expenses are anticipated to be approximately $4.5 billion.
Selling, General and Administrative Expenses: Selling, general and administrative expenses for 2024 are projected to be approximately $1.3 billion.
Income Taxes: The Company continues to expect its full-year tax expense to be negligible.
Capital Expenditures: Capital expenditures for 2024 are expected to be approximately $0.9 billion.
Cash and Investments: Year-end cash and investments for 2024 are projected to be approximately $9 billion.
Recent Progress and Upcoming Late-Stage Pipeline Milestones
With 10 late-stage programs, and additional new programs advancing toward pivotal studies, Moderna continues to advance its pipeline and expects numerous product milestones in 2024 across its vaccines and therapeutics portfolio. Late-stage includes eight programs that have progressed to Phase 3 clinical studies and two rare disease programs that are moving toward registrational studies.
Respiratory vaccines:
Respiratory syncytial virus (RSV) vaccine: Moderna's RSV vaccine candidate (mRNA-1345) is in an ongoing Phase 2/3 clinical trial, ConquerRSV. Based on positive data from the study, Moderna has filed for regulatory approvals for the prevention of RSV-associated lower respiratory tract disease (RSV-LRTD) and acute respiratory disease (ARD) in adults over 60 years of age. As mentioned above, the Company is awaiting regulatory approvals and the U.S. ACIP recommendation in 2024.
At its Vaccines Day event in March, Moderna announced the initiation of multiple Phase 3 expansion studies of its RSV vaccine in adults over 50 years of age to evaluate co-administration and revaccination. Additional trials (Phase 1 - Phase 3) have been initiated for high-risk adults, as well as maternal and pediatric populations. Interim data from these studies could be available as early as 2024.
Seasonal flu vaccine: Moderna's seasonal flu vaccine (mRNA-1010) demonstrated consistently acceptable safety and tolerability across three Phase 3 trials. In the most recent Phase 3 trial (P303), mRNA-1010 met all immunogenicity endpoints, demonstrating higher titers compared to a currently licensed standard-dose flu vaccine. In an older adult extension study of P303, mRNA-1010 is being studied against high dose Fluzone HD®; the trial is fully enrolled. The Company is in ongoing discussions with regulators and intends to 2024.
Next-generation COVID-19 vaccine: A recent announcement of positive interim results from the NEXTCove Phase 3 trial showed that Moderna's next-generation COVID-19 vaccine (mRNA-1283) elicited a higher immune response against both the Omicron BA.4/BA.5 and original virus strains of SARS-CoV-2 compared to Moderna's licensed COVID-19 vaccine (mRNA-1273.222). The next-generation vaccine is designed to be refrigerator-stable and paves the way for a combination vaccine against influenza and COVID-19 (mRNA-1083). This is Moderna's fourth infectious disease vaccine program with positive Phase 3 data. The Company is engaging with regulators on next steps.
Seasonal flu + COVID vaccine: Moderna's Phase 3 trial of its combination vaccine against seasonal flu and COVID-19 (mRNA-1083) is fully enrolled. The Company anticipates data from the study in 2024.
Latent and other vaccines:
Cytomegalovirus (CMV) vaccine: The pivotal Phase 3 study of Moderna's CMV vaccine candidate (mRNA-1647) is fully enrolled and accruing cases, evaluating its efficacy, safety and immunogenicity in the prevention of primary infection in women of childbearing age. The first interim analysis for the evaluation of vaccine efficacy is expected as early as the end of 2024.
Epstein-Barr virus (EBV) vaccine: Moderna's EBV vaccine candidate for the prevention of infectious mononucleosis (mRNA-1189) showed positive immunogenicity and safety data in a Phase 1 study. The Company is advancing this candidate toward pivotal development.
Varicella-Zoster virus (VZV) vaccine: In a Phase 1/2 trial, Moderna's VZV vaccine candidate for the prevention of shingles (mRNA-1468) elicited comparable or higher T cell responses relative to Shingrix. The Company is advancing toward a pivotal Phase 3 trial.
Herpes simplex virus (HSV) vaccine: The Phase 1/2 study of Moderna's HSV vaccine candidate (mRNA-1608) is fully enrolled.
Norovirus vaccine: Moderna's vaccine candidate for the prevention of norovirus (mRNA-1403) showed positive immunogenicity and safety data in a Phase 1 study. The Company is advancing toward a pivotal Phase 3 trial.
Oncology therapeutics:
Individualized Neoantigen Therapy (INT): Moderna continues to demonstrate the potential clinical benefit of its INT program (mRNA-4157). In partnership with Merck, two Phase 3 trials in resected high-risk (stage III/IV) melanoma and completely resected stage II, IIIA or IIIB non-small cell lung cancer are ongoing.
Moderna and Merck have initiated three new randomized clinical studies in additional tumor types in 2024, including a Phase 2 adjuvant treatment in patients with renal cell carcinoma, or kidney cancer; a Phase 2 adjuvant treatment in patients with high-risk muscle-invasive bladder cancer; and a Phase 2/3 neoadjuvant and adjuvant treatment in patients with cutaneous squamous cell carcinoma, the second most common form of skin cancer.
Rare disease and other therapeutics:
Propionic acidemia (PA) & methylmalonic acidemia (MMA) therapeutics: Interim data for a first-in-human, Phase 1/2, open-label, dose optimization study and extension study, evaluating the safety and efficacy of an investigational mRNA therapy for PA (mRNA-3927), was published in Nature. The Company expects to advance its PA and MMA (mRNA-3705) programs into registrational studies in 2024.
PD-L1 therapeutic: Following a strategic review, and as a result of its decision to prioritize investments in other programs, Moderna is discontinuing development of its preclinical PD-L1 program (mRNA-6981), and is no longer evaluating other mRNA candidates in this area.
Moderna Corporate Updates
Announced the advancement of multiple vaccine programs to late-stage clinical trials at its fifth Vaccines Day Investor Event on March 27, 2024.
Announced a development and commercialization funding agreement on March 27, 2024, with Blackstone Life Sciences for up to $750 million to advance Moderna's flu program.
Announced a new collaboration with OpenAI to advance mRNA medicine using generative AI.
Entered into a non-exclusive intellectual property licensing agreement with an upfront payment and low double-digit royalty on the net sales of a COVID-19 product from a pharmaceutical company in the territory of Japan.
Agreed with Metagenomi to terminate gene editing collaboration as Moderna continues to strategically prioritize its research and development investments.
The Moderna Annual Meeting of Shareholders will be held on May 6, 2024, at 8:00 a.m. ET.
Company Accolade
Moderna was named for the first time to the LinkedIn Top Companies list of the best workplaces to grow a career in the U.S.
Key 2024 Investor and Analyst Event Dates
ASCO Investor Event: June 3
R&D Day: September 12
Investor Call and Webcast Information
Moderna will host a live conference call and webcast at 8:00 a.m. ET on May 2, 2024. To access the live conference call via telephone, please register at the link below. Once registered, dial-in numbers and a unique pin number will be provided. A live webcast of the call will also be available under "Events and Presentations" in the Investors section of the Moderna website.
Telephone:
Webcast:
The archived webcast will be available on Moderna's website approximately two hours after the conference call and will be available for one year following the call.
About Moderna
Moderna is a leader in the creation of the field of mRNA medicine. Through the advancement of mRNA technology, Moderna is reimagining how medicines are made and transforming how we treat and prevent disease for everyone. By working at the intersection of science, technology and health for more than a decade, the company has developed medicines at unprecedented speed and efficiency, including one of the earliest and most effective COVID-19 vaccines.
Moderna's mRNA platform has enabled the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases and autoimmune diseases. With a unique culture and a global team driven by the Moderna values and mindsets to responsibly change the future of human health, Moderna strives to deliver the greatest possible impact to people through mRNA medicines. For more information about Moderna, please visit modernatx.com and connect with us on X (formerly Twitter), Facebook, Instagram, YouTube and LinkedIn.
MODERNA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited, in millions, except per share data)
Three Months Ended March 31,
2024
2023
Revenue:
Net product sales
$
167
$
1,828
Other revenue1
-
34
Total revenue
167
1,862
Operating expenses:
Cost of sales
96
792
Research and development
1,063
1,131
Selling, general and administrative
274
305
Total operating expenses
1,433
2,228
Loss from operations
(1,266
)
(366
)
Interest income
120
109
Other expense, net
(19
)
(48
)
Loss before income taxes
(1,165
)
(305
)
Provision for (benefit from) income taxes
10
(384
)
Net (loss) income
$
(1,175
)
$
79
(Loss) earnings per share:
Basic
$
(3.07
)
$
0.20
Diluted
$
(3.07
)
$
0.19
Weighted average common shares used in calculation of (loss) earnings per share:
Basic
382
386
Diluted
382
405
_______
1Includes grant revenue and collaboration revenue
MODERNA, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(Unaudited, in millions)
March 31,
December 31,
2024
2023
Assets
Current assets:
Cash and cash equivalents
$
2,051
$
2,907
Investments
6,472
5,697
Accounts receivable, net
137
892
Inventory
295
202
Prepaid expenses and other current assets
645
627
Total current assets
9,600
10,325
Investments, non-current
3,638
4,677
Property, plant and equipment, net
2,063
1,945
Right-of-use assets, operating leases
697
713
Deferred tax assets
81
81
Other non-current assets
650
685
Total assets
$
16,729
$
18,426
Liabilities and Stockholders' Equity
Current liabilities:
Accounts payable
$
183
$
520
Accrued liabilities
1,396
1,798
Deferred revenue
559
568
Income taxes payable
52
63
Other current liabilities
190
66
Total current liabilities
2,380
3,015
Deferred revenue, non-current
58
83
Operating lease liabilities, non-current
637
643
Financing lease liabilities, non-current
575
575
Other non-current liabilities
262
256
Total liabilities
3,912
4,572
Stockholders' equity:
Additional paid-in capital
487
371
Accumulated other comprehensive loss
(101
)
(123
)
Retained earnings
12,431
13,606
Total stockholders' equity
12,817
13,854
Total liabilities and stockholders' equity
$
16,729
$
18,426
MODERNA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited, in millions)
Three Months Ended March 31,
2024
2023
Operating activities
Net (loss) income
$
(1,175
)
$
79
Adjustments to reconcile net (loss) income to net cash used in operating activities:
Stock-based compensation
101
75
Depreciation and amortization
36
78
Amortization/accretion of investments
(27
)
(17
)
Loss on equity investments, net
13
18
Deferred income taxes
-
(310
)
Other non-cash items
3
(4
)
Changes in assets and liabilities, net of acquisition of business:
Accounts receivable, net
755
272
Prepaid expenses and other assets
3
(212
)
Inventory
(93
)
216
Right-of-use assets, operating leases
16
4
Accounts payable
(303
)
(117
)
Accrued liabilities
(398
)
(495
)
Deferred revenue
(33
)
(819
)
Income taxes payable
(11
)
18
Operating lease liabilities
(6
)
4
Other liabilities
130
(15
)
Net cash used in operating activities
(989
)
(1,225
)
Investing activities
Purchases of marketable securities
(2,544
)
(1,085
)
Proceeds from maturities of marketable securities
1,573
1,360
Proceeds from sales of marketable securities
1,285
1,957
Purchases of property, plant and equipment
(196
)
(113
)
Acquisition of business, net of cash acquired
-
(85
)
Investment in convertible notes and equity securities
-
(23
)
Net cash provided by investing activities
118
2,011
Financing activities
Proceeds from issuance of common stock through equity plans
15
9
Repurchase of common stock, including excise tax
-
(526
)
Changes in financing lease liabilities
(1
)
(25
)
Net cash provided by (used in) financing activities
14
(542
)
Net (decrease) increase in cash, cash equivalents and restricted cash
(857
)
244
Cash, cash equivalents and restricted cash, beginning of year
2,928
3,217
Cash, cash equivalents and restricted cash, end of period
$
2,071
$
3,461
Spikevax® is a registered trademark of Moderna.
Fluzone® is a registered trademark of Sanofi Pasteur.
KEYTRUDA® is a registered trademark of Merck Sharp & Dohme Corp.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding: Moderna's anticipated approval and launch of its RSV vaccine, market dynamics, and its competitive profile; Moderna's 2024 financial framework and anticipated performance, including expected revenues; and anticipated milestones for Moderna's pipeline programs in 2024. In some cases, forward-looking statements can be identified by terminology such as "will," "may," "should," "could," "expects," "intends," "plans," "aims," "anticipates," "believes," "estimates," "predicts," "potential," "continue," or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna's control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties, and other factors include, among others, those risks and uncertainties described under the heading "Risk Factors" in Moderna's Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the U.S. Securities and Exchange Commission (SEC), and in subsequent filings made by Moderna with the SEC, which are available on the SEC's website at . Except as required by law, Moderna disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release in the event of new information, future developments or otherwise. These forward-looking statements are based on Moderna's current expectations and speak only as of the date of this press release.
###
Moderna Contacts
Media:
Chris Ridley
Head of Global Media Relations
+1 617-800-3651
Chris.Ridley@modernatx.com
Investors:
Lavina Talukdar
Senior Vice President & Head of Investor Relations
+1 617-209-5834
Lavina.Talukdar@modernatx.com
SOURCE: Moderna, Inc.
View the original press release on accesswire.com
VaccinePhase 3Clinical ResultFinancial StatementPhase 1
16 Oct 2023
Experts at Cincinnati Children's and Children's Hospital Colorado say findings could enable more at-risk children to be protected from measles, mumps, and chicken pox
CINCINNATI, Oct. 16, 2023 /PRNewswire/ -- Live vaccinations provided to children who previously received liver or kidney transplants were found to be safe and prompted an immune response to guard against several life-threatening conditions, according to a new study published Oct. 12, 2023, in JAMA Network Open.
Continue Reading
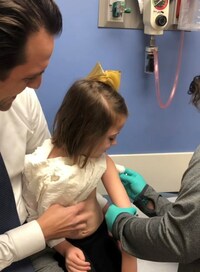
A liver-transplant recipient receives the MMR vaccine at Cincinnati Children’s. The study involving 18 U.S. institutions that perform organ transplants included 281 children on chronic immunosuppressive medications. (Credit: Family provided photo)
The study, based on data from 18 organ transplant centers, was co-authored by Lara Danziger-Isakov, MD, MPH, interim director of the Division of Infectious Diseases at Cincinnati Children's, and Amy Feldman, MD, MSCS, medical director of the Liver Transplant Program at Children's Hospital Colorado.
The results are important because the rates of measles, mumps and the varicella-zoster virus that causes chicken pox are rising nationally and internationally, leaving immunocompromised children at risk for life-threatening conditions, according to Danziger-Isakov, senior author of the study.
"This shifts the paradigm of the approach to protecting this vulnerable population where live viral vaccines were previously avoided," Danziger-Isakov says. "This should enable children who have received organ transplants to integrate into their communities with more confidence and decreased risk for acquiring chicken pox or measles, which have both made a resurgence."
Global surges of measles and mumps were exacerbated by decreasing herd immunity as millions of vaccine doses were missed during the COVID-19 pandemic, leaving nonimmune organ transplant recipients at significant risk for community exposure, infection, disease and death from wild-type infection, says Feldman, first author of the study.
"Community acquisition of measles and varicella is a real risk for immunocompromised children in today's world," Feldman says. "These infections can be fatal in transplant recipients. Being able to administer live vaccines post-transplant, and provide immunity, is critical."
Historically, live vaccines to guard against measles, mumps, rubella (MMR) and varicella-zoster virus (VZV) have not been recommended after solid organ transplant due to concerns about a theoretical risk of vaccine strain infection in immunocompromised children. However, the authors reported no serious adverse events observed following the live vaccination of the children enrolled in the trial between Jan. 1, 2002, and Feb. 28, 2023.
Transplant recipients who participated in the trial received one to three doses of MMR vaccine and/or one to three doses of VZV vaccine. The cohort included 281 children on chronic immunosuppressive medications with a median age of 8.9 years at the time of the first post-transplant vaccine. The median time from transplant to enrollment in the study was 6.3 years. Safety data were collected after each vaccination, and antibody levels were measured at zero to three months and one year after vaccination.
The majority of children developed protective antibodies following vaccination – 72% for varicella, 86% for measles, 83% for mumps and 99% for rubella. One year after vaccination, the majority of children who developed protective antibodies maintained that protection – 77% for varicella, 92% for measles, 83% for mumps and 94% for rubella. Five children developed clinical varicella, but all of their conditions resolved within one week.
The findings suggest that live vaccinations may be safe and immunogenic after solid organ transplant in select pediatric recipients and can offer protection against circulating measles, mumps and varicella. Further research is needed to understand long-term maintenance of immunity after vaccination of children who receive organ transplants, as well as factors associated with immune response and clinical protection, the authors noted.
Feldman reported that she is funded by a K08 grant (HS026510) from the Agency for Healthcare Research and Quality.
Danziger-Isakov reported receiving clinical research support paid to her institution from AiCuris, Ansun Biopharma, Astellas Pharma, Merck, Pfizer and Takeda; advisory board fees from GlaxoSmithKline and Roche; and consultant fees from Merck outside the submitted work.
About Cincinnati Children's
Cincinnati Children's ranks No. 1 in the nation in U.S. News & World Report's 2023-24 listing of Best Children's Hospitals. In addition, Cincinnati Children's is recognized as one of America's Most Innovative Companies by Fortune. Nearly one-third of the health system's 18,500 employees are engaged in research, and Cincinnati Children's is one of the top two recipients of pediatric research grants from the National Institutes of Health. Additional information may be found at
SOURCE Cincinnati Children's Hospital Medical Center
VaccineClinical ResultClinical Study
100 Deals associated with VZV vaccine(Merck Sharp & Dohme Corp.)
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Hematologic Neoplasms | Phase 3 | - | 24 Jun 2011 | |
| Malignant Solid Neoplasm | Phase 3 | - | 24 Jun 2011 | |
| Herpes Zoster | Phase 3 | United States | 01 Nov 1998 | |
| Autoimmune Diseases | Phase 2 | - | 21 Feb 2012 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 1 | 290 | (Part 1: Gamma- Irradiated VZV Vaccine A) | cqednylkyq(majvwginem) = uszhdggdhw enmrkcwgmy (fghieecmuf, gnbarkikzf - knbvjhasfc) View more | - | 08 Nov 2019 | ||
(Part 1: Heat-treated Varicella-Zoster Virus (VZV) Vaccine) | eqfjczqpmm = kwehwmlzdp lmoylwmewg (ezassvnnqx, kjyykwuerl - ppwgnjpaib) View more | ||||||
Phase 1 | 80 | vzvvbdfywa(sibwujfucz) = xejggzipmr yitncckrgn (liezabhgii, cdkdipqedk - knfdnncysh) View more | - | 11 Mar 2019 | |||
Phase 2 | 354 | (V212) | qqqaozincn(nzrhwnimhf) = aqhmbagqbo yqkoskpmhf (auwxsarwfn, jkqwkwqrus - vsdxlcqowt) View more | - | 14 Jan 2019 | ||
Placebo (Placebo) | kenqeuqclo = wouhyemklb kqwamvqbns (ufjwrsvkyb, zlsahgasal - jmpwrkmatn) View more | ||||||
Phase 3 | 1,257 | Matching placebo | acfedgadej = whtderaibi gfzsehonpf (mvpkgueosg, auqrkbkclx - pbucjodnrm) View more | - | 02 Jul 2018 | ||
Phase 3 | 5,305 | (V212-STM) | tvmnodnkau = adqayjoruf rfjndvjbzj (rtuvebndyt, kfnfrbpusp - bjpaycdqhe) View more | - | 13 Apr 2018 | ||
Placebo (Placebo-STM) | tvmnodnkau = xvnmaxvryo rfjndvjbzj (rtuvebndyt, fgfnszyfqa - bdjwrtnxyd) View more |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
Deal
Boost your decision using our deal data.
login
or
Core Patent
Boost your research with our Core Patent data.
login
or
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free

