Request Demo
Last update 14 Dec 2025
Bepirovirsen
Last update 14 Dec 2025
Overview
Basic Info
Drug Type ASO |
Synonyms Bepirovirsen Sodium, IONIS HBVRx, IONIS-HBVRx + [10] |
Target |
Action inhibitors |
Mechanism HBsAg inhibitors(HBsAg inhibitors) |
Therapeutic Areas |
Active Indication |
Inactive Indication- |
Originator Organization |
Active Organization |
Inactive Organization |
License Organization |
Drug Highest PhasePhase 3 |
First Approval Date- |
RegulationFast Track (United States), Breakthrough Therapy (China) |
Login to view timeline
Structure/Sequence
Boost your research with our RNA technology data.
login
or
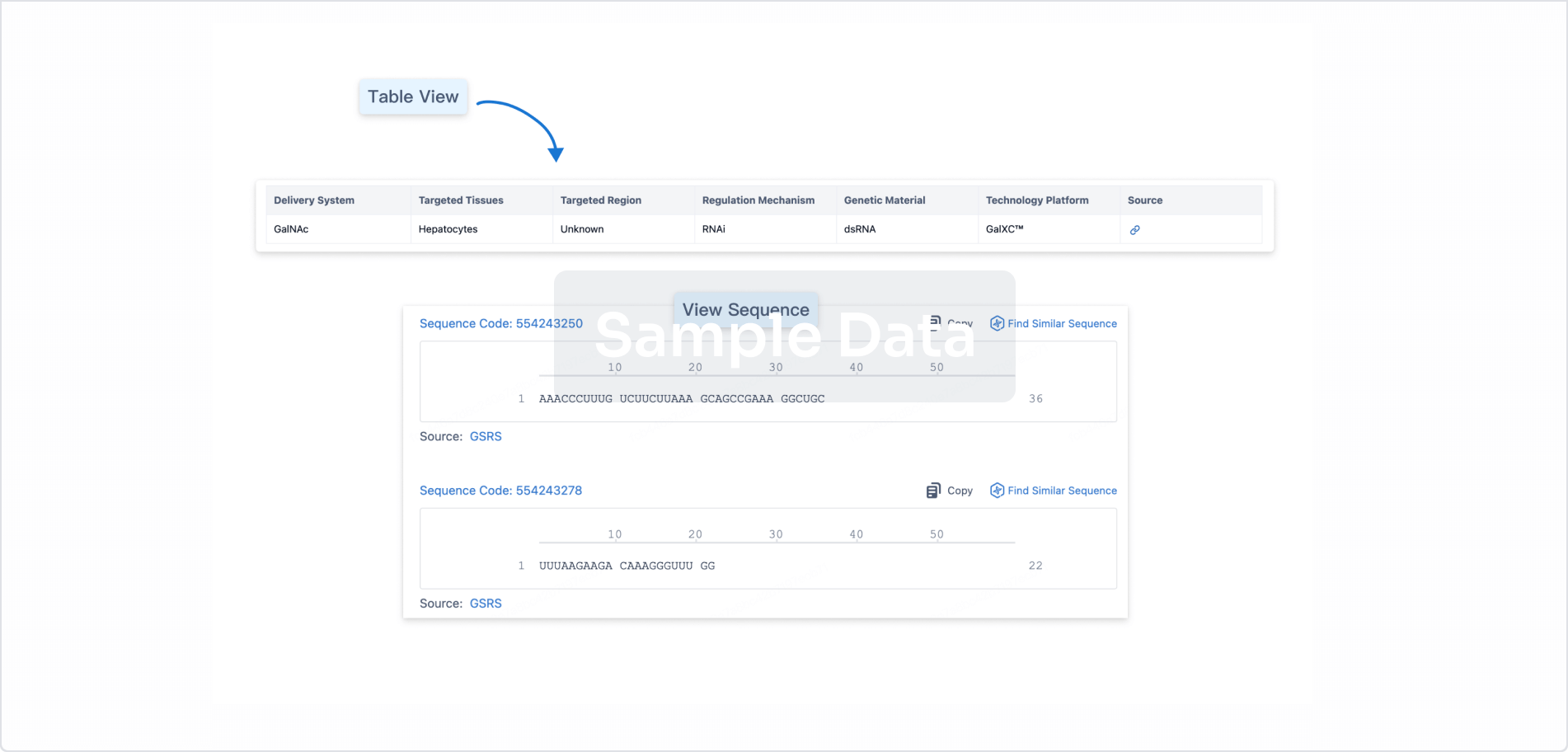
Sequence Code 31829872

Source: *****
Related
21
Clinical Trials associated with BepirovirsenNCT07168356
A Phase 1, Open-Label, Single-Dose, Parallel Group, 2-Part Study to Evaluate the Pharmacokinetics of Bepirovirsen in Adult Participants With Severe or Moderate Renal Impairment Compared to Matched Healthy Control Participants
This is a 2-part study where all participants will receive bepirovirsen. The study will measure how much bepirovirsen from a single dose gets into the blood, and how long it stays in the blood. The purpose of the study is to understand if blood levels of bepirovirsen are different in adults with kidney disease compared to healthy adults who do not have kidney disease.
Start Date16 Sep 2025 |
Sponsor / Collaborator |
CTRI/2025/03/083590
A prospective, multi-centre study (B-Sure) to evaluate long-term durability of treatment response in chronic hepatitis B participants with and without nucleos(t)ide therapy who have participated in a previous bepirovirsen treatment study - B-Sure
Start Date08 Apr 2025 |
Sponsor / Collaborator |
NCT06537414
A Phase 2b, Multi-centre, Randomized, Partially Placebo-controlled, Double-blind Study to Investigate the Safety and Efficacy of Sequential Therapy With Daplusiran/Tomligisiran Followed by Bepirovirsen in Participants With Chronic Hepatitis B Virus on Background Nucleos(t)Ide Analogue Therapy (B-United)
The study is intended to evaluate the efficacy and safety of 2 different doses of DAP/TOM followed by bepirovirsen in participants living with CHB on standard of care nucleos(t)ide analogue (NA) therapy. The study also aims to identify an optimal dose of DAP/TOM for sequenced therapy with bepirovirsen for further clinical development and to assess the contribution of DAP/TOM to the sequential regimen.
Start Date11 Nov 2024 |
Sponsor / Collaborator |
100 Clinical Results associated with Bepirovirsen
Login to view more data
100 Translational Medicine associated with Bepirovirsen
Login to view more data
100 Patents (Medical) associated with Bepirovirsen
Login to view more data
27
Literatures (Medical) associated with Bepirovirsen01 Oct 2025·Clinical Drug Investigation
Emerging Trends and Future Directions in the Development of Anti-hepatitis B Therapies: A Horizon Scanning Review
Review
Author: Wu, Bin ; Yan, Mengxia ; Bao, Yun ; Feng, Hao ; Yao, Kaijie ; Chen, Ying ; Li, Wen
BACKGROUND AND OBJECTIVES:
Chronic hepatitis B virus infection remains a Major global public health challenge, affecting over 254 million individuals and causing substantial mortality owing to the limited curative potential of current therapies. This horizon scanning review aims to comprehensively analyze emerging trends and future directions in novel anti-hepatitis B virus therapeutic development, evaluating their progress and potential to achieve functional or complete cures.
METHODS:
We conducted a systematic horizon scanning review from January 2020 to June 2025, searching databases (PubMed, Embase, the Cochrane Library, China National Knowledge Infrastructure [CNKI], and WanFang), clinical trial registries (ClinicalTrials.gov, EU Clinical Trials Register, World Health Organization International Clinical Trials Registry, Chinese Clinical Trial Registry, and chinadrugtrials.org), hepatology conference abstracts (American Association for the Study of Liver Diseases, European Association for the Study of the Liver), and pharmaceutical company websites. Inclusion criteria focused on studies detailing novel anti-hepatitis B virus treatments, with data extracted on category, target, clinical phase, and discontinuation reasons.
RESULTS:
Our analysis identified 161 unique anti-hepatitis B virus treatments: 75 in clinical trials, 34 in preclinical development, and 52 discontinued post-clinical trials because of safety or insufficient efficacy. The pipeline reveals a diversification of targets, with capsid assembly modulators, therapeutic vaccines, and monoclonal antibodies being most prevalent. Three therapies representing novel mechanisms have reached phase III-bepirovirsen (an antisense oligonucleotide), canocapavir (a capsid assembly modulator), and εPA-44 (a therapeutic vaccine), illustrating diversification of late-stage pipelines; however, 6-month off-treatment endpoints should be interpreted cautiously across modalities until durability is established.
CONCLUSIONS:
The robust and diverse pipeline of novel anti-hepatitis B virus treatments offers promise for improving functional cure rates, but definitive conclusions await longer off-treatment follow-up and durability data. Continued research, rational combination strategies, and global collaboration are crucial to overcome challenges and ensure equitable access to these transformative therapies worldwide.
01 Sep 2025·Current Opinion in HIV and AIDS
Advancing HIV cure: insights from developing chronic hepatitis b therapies for functional cure
Review
Author: Verma, Ana ; Chung, Raymond T
Purpose of review:
Similarly to HIV, HBV assumes a highly stable nuclear form and becomes integrated into the host genome, posing a significant challenge to complete eradication. The purpose of this review is to highlight the recent progress on various therapies that are being explored to achieve functional cure (FC) of chronic Hepatitis B (CHB).
Recent findings:
The current standard-of-care for CHB is either nucleos(t)ide analogues (NA) or PegIFN-α, but neither alone is sufficient to achieve functional cure. However, NA cessation alone or followed by PegIFN-α shows promise for increasing functional cure rates and decreasing viral relapse rates. While first generation capsid-assembly modulators (CAMs) had virtually no impact on HBsAg, newer, more potent CAMs may have an effect on cccDNA and produce reductions in HBsAg levels. Small-interfering RNAs (siRNAs) can lower HBsAg, but do not appear to result in sustained HBsAg clearance. A similar agent, bepirovirsen (an antisense oligonucleotide), appears to be more effective in producing modest FC rates; this may be due to its possible induction of the innate immune response.
Summary:
Given the persistence of cccDNA and integrated DNA, together with HBsAg-induced immune dysfunction, successful treatment for CHB to induce FC is likely to require a combination of agents that inhibit viral replication, reduce HBsAg levels, and boost the antiviral immune response.
01 Feb 2025·JOURNAL OF HEPATOLOGY
Sequential Peg-IFN after bepirovirsen may reduce post-treatment relapse in chronic hepatitis B
Article
Author: Plesniak, Robert ; Gordon, Stuart C. ; Coffin, Carla S ; Chak, Eric ; Dixon, Susan ; Morozov, Vyacheslav ; Ryan, Pablo ; Felton, Leigh ; Voloshina, Natalya ; Gankina, Natalya ; Gordon, Stuart C ; Kendrick, Stuart ; Lim, Jessica ; Komori, Atsumasa ; Andreone, Pietro ; Plein, Helene ; Yim, Hyung Joon ; Youssef, Amir S ; Theodore, Dickens ; McGonagle, Grant ; Greene, Thomas ; Tanaka, Yasuhito ; Sagalova, Olga ; Buti, Maria ; Coffin, Carla S. ; McPherson, Stuart ; Lampertico, Pietro ; Janczewska, Ewa ; Cabezas, Joaquín ; Xie, Qing ; Lakshminarayanan, Divya ; Elston, Rob ; Atsukawa, Masanori ; Sheng, Guoping ; Heo, Jeong ; Fujiwara, Kei ; Youssef, Amir ; Paff, Melanie ; Poulin, Sébastien ; Lee, Maximilian
BACKGROUND & AIMS:
Bepirovirsen, an antisense oligonucleotide, induces sustained reductions in hepatitis B surface antigen (HBsAg) and HBV DNA to below the lower limit of quantification (
METHODS:
In this phase IIb, multicentre, open-label trial, participants on stable nucleos(t)ide analogue (NA) therapy were randomised 1:1 to bepirovirsen 300 mg once weekly (plus loading dose on Days 4 and 11) for 24 (Arm 1) or 12 (Arm 2) weeks followed by Peg-IFN 180 μg once weekly for up to 24 weeks, with up to 36 weeks follow-up. Participants continued NA therapy throughout. The primary outcome was the proportion of participants with HBsAg <0.05 IU/ml and HBV DNA
RESULTS:
The intent-to-treat population included 108 participants (Arm 1, n = 55; Arm 2, n = 53). The primary outcome was achieved by 5 (9%) participants in Arm 1 and 8 (15%) in Arm 2. All responders had baseline HBsAg ≤3,000 IU/ml. Indirect comparison with the phase IIb study B-Clear indicates that sequential addition of Peg-IFN may reduce the relapse rates previously observed with bepirovirsen alone. The proportions of participants with adverse events and treatment-related adverse events in both treatment windows were similar between treatment arms.
CONCLUSIONS:
Sequential therapy with bepirovirsen followed by Peg-IFN is tolerable and effective in participants with chronic HBV infection on stable NA therapy. This proof-of-concept trial demonstrates a potential strategy to extend responses to bepirovirsen by reducing relapse.
IMPACT AND IMPLICATIONS:
This phase IIb study investigated whether sequential therapy with bepirovirsen followed by Peg-IFN could improve off-treatment response rates to bepirovirsen alone by converting partial bepirovirsen responders to full responders and/or reducing relapse rates in participants with chronic HBV. These data show that sequential therapy with bepirovirsen followed by Peg-IFN is tolerable and effective; in patients with a bepirovirsen response, sequential treatment with Peg-IFN may help to reduce off-treatment relapses in participants on stable NA. Participants had a similar response during bepirovirsen treatment as seen in B-Clear, with increased response rates in participants with lower baseline HBsAg; all responders to the sequential regimen had baseline HBsAg <3000 IU/mL. As the first study of antisense oligonucleotide-mediated RNA silencing followed by interferon immunomodulation in patients with chronic HBV infection, this study is an important proof-of-concept for sequential therapy, shedding light on the therapeutic potential of utilising immunomodulators following suppression of HBV antigens.
CLINICAL TRIAL NUMBER:
NCT04676724.
60
News (Medical) associated with Bepirovirsen29 Oct 2025
- TRYNGOLZA® generated $32 million in net product sales in the third quarter 2025 -
- DAWNZERA™ (donidalorsen) launch off to encouraging start -
- Olezarsen significantly reduced triglycerides and acute pancreatitis events in severe hypertriglyceridemia (sHTG) in landmark Phase 3 studies; sNDA submission on track by year-end –
- Positive pivotal zilganersen results in Alexander disease position Ionis for first independent neurology launch in 2026 -
- Increasing 2025 financial guidance driven by continued strength across the business –
CARLSBAD, Calif. --(BUSINESS WIRE)--Oct. 29, 2025-- Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) (the “Company”) today reported financial results and provided key updates for the third quarter ended September 30, 2025 .
"The third quarter was a watershed moment for Ionis, as we made important progress advancing our Ionis-owned medicines. With two independent launches now underway, and two more anticipated in 2026, we are delivering on our goal to bring a steady cadence of new medicines to people in need,” said Brett P. Monia , Ph.D., chief executive officer of Ionis. “Last month, we announced groundbreaking, positive topline Phase 3 results for olezarsen in severe hypertriglyceridemia and for zilganersen in Alexander disease, with regulatory filings planned in the coming months. Our approved and late-stage portfolio continues to deliver — positioning Ionis for substantial growth while, most importantly, offering the opportunity to profoundly improve the lives of people with serious diseases."
Third Quarter 2025 Summary Financial Results(1):
Three months ended
Nine months ended
September 30 ,
September 30 ,
2025
2024
2025
2024
(amounts in millions)
Total revenue
$
157
$
134
$
740
$
479
Operating expenses
$
317
$
282
$
907
$
843
Operating expenses on a non-GAAP basis
$
286
$
250
$
816
$
749
Loss from operations
($
160
)
($
148
)
($
167
)
($
364
)
Loss from operations on a non-GAAP basis
($
129
)
($
116
)
($
76
)
($
270
)
(1)
Reconciliation of GAAP to non-GAAP basis contained later in this release.
Recent Financial Highlights
Revenue increased 17% in the third quarter of 2025 and increased 55% in the nine months ended September 30, 2025 , compared to the same periods last year, driven by the continued successful launch of TRYNGOLZA and increased royalty revenues. Contributing to the year-to-date increase, Ionis earned a $280 million upfront payment for the global license of sapablursen to Ono Pharmaceutical Co., Ltd. in the second quarter of 2025 Operating expenses on a non-GAAP basis increased 14% in the third quarter of 2025 and increased 9% in the nine months ended September 30, 2025 , compared to the same periods last year, primarily due to investments related to commercialization efforts for TRYNGOLZA, DAWNZERA and WAINUA Increasing 2025 financial guidance reflects strong overall revenue performance experienced year-to-date and fourth quarter outlook, including strong momentum seen with TRYNGOLZA revenues:
Full Year 2025 Guidance
Previous Guidance
New Guidance
Total Revenue
$825-850 million
$875-900 million
TRYNGOLZA product sales, net
$75-80 million
$85-95 million
Operating loss on a non-GAAP basis
$300-325 million
$275-300 million
Cash, cash equivalents and short-term investments
~$2.0 billion
> $2.1 billion
Third Quarter 2025 Financial Results
"In the third quarter of 2025, we delivered strong revenue performance, highlighted by TRYNGOLZA’s nearly 70% increase over the prior quarter. As a result of this strength and our fourth quarter outlook, we are increasing our financial guidance again for 2025," said Elizabeth L. Hougen , chief financial officer of Ionis. "Looking ahead, we expect the 2026 independent launches of olezarsen in severe hypertriglyceridemia and zilganersen in Alexander disease to further strengthen our commercial portfolio. We anticipate that growth in our product revenues coupled with additional partner revenues will position Ionis to achieve cash flow breakeven in 2028 and generate substantial and sustainable positive cash flow for years to come."
Recent Highlights - Wholly Owned Medicines
TRYNGOLZA® (olezarsen), the first and only FDA approved treatment for adults living with familial chylomicronemia syndrome (FCS) as an adjunct to diet Generated net product sales of $32 million in the third quarter of 2025, its third full quarter on the market, a nearly 70% increase over the prior quarter, and $57 million in the nine months ended September 30, 2025 Approved in the European Union (EU) as an adjunct to diet in adult patients for the treatment of genetically confirmed FCS ; Sobi anticipates launching in the fourth quarter 2025 Olezarsen demonstrated positive topline results in the pivotal Phase 3 CORE and CORE2 studies in sHTG Olezarsen demonstrated a highly statistically significant placebo-adjusted mean reduction in fasting triglycerides of up to 72% and a highly statistically significant reduction in acute pancreatitis events of 85% with favorable safety and tolerability sNDA submission on track for the end of 2025 with approval anticipated in the fourth quarter of 2026 Detailed data to be presented at the American Heart Association Conference on November 8, 2025 , in a late-breaking session DAWNZERA™ (donidalorsen) was approved on August 21, 2025 , by the FDA for prophylaxis to prevent attacks of hereditary angioedema ( HAE) in adult and pediatric patients 12 years of age and older First and only RNA-targeted prophylactic therapy that has the potential to offer durable efficacy, a favorable safety and tolerability profile, and the longest available dosing interval, with self-administration via autoinjector every four or eight weeks U.S. launch underway and off to an encouraging start Currently under regulatory review in the EU Zilganersen demonstrated positive results in the pivotal study in children and adults with Alexander disease (AxD), a rare, progressive, and often fatal neurological disorder with no approved disease-modifying treatments Zilganersen 50 mg demonstrated statistically significant and clinically meaningful stabilization on the primary endpoint of gait speed as assessed by the 10-Meter Walk Test (10MWT), compared to control at week 61 (mean difference 33.3%) with favorable safety and tolerability Additional data from the pivotal study in children and adults living with Alexander disease were presented at the Child Neurology Society Annual Meeting in October 2025 NDA submission planned for the first quarter of 2026 with approval anticipated next year ION582 granted Breakthrough Therapy designation from FDA for the treatment of Angelman syndrome Phase 3 REVEAL study expected to be fully enrolled in 2026 Phase 2 HALOS study showed continued improvement across multiple functional measures versus natural history through 18 months including expressive communication
Generated net product sales of $32 million in the third quarter of 2025, its third full quarter on the market, a nearly 70% increase over the prior quarter, and $57 million in the nine months ended September 30, 2025 Approved in the European Union (EU) as an adjunct to diet in adult patients for the treatment of genetically confirmed FCS ; Sobi anticipates launching in the fourth quarter 2025
Olezarsen demonstrated a highly statistically significant placebo-adjusted mean reduction in fasting triglycerides of up to 72% and a highly statistically significant reduction in acute pancreatitis events of 85% with favorable safety and tolerability sNDA submission on track for the end of 2025 with approval anticipated in the fourth quarter of 2026 Detailed data to be presented at the American Heart Association Conference on November 8, 2025 , in a late-breaking session
First and only RNA-targeted prophylactic therapy that has the potential to offer durable efficacy, a favorable safety and tolerability profile, and the longest available dosing interval, with self-administration via autoinjector every four or eight weeks U.S. launch underway and off to an encouraging start Currently under regulatory review in the EU
Zilganersen 50 mg demonstrated statistically significant and clinically meaningful stabilization on the primary endpoint of gait speed as assessed by the 10-Meter Walk Test (10MWT), compared to control at week 61 (mean difference 33.3%) with favorable safety and tolerability Additional data from the pivotal study in children and adults living with Alexander disease were presented at the Child Neurology Society Annual Meeting in October 2025 NDA submission planned for the first quarter of 2026 with approval anticipated next year
Phase 3 REVEAL study expected to be fully enrolled in 2026 Phase 2 HALOS study showed continued improvement across multiple functional measures versus natural history through 18 months including expressive communication
Recent Highlights – Partnered Medicines
WAINUA® (eplontersen) (WAINZUA in EU) for the treatment of adults with polyneuropathy of hereditary transthyretin-mediated amyloidosis (ATTRv-PN) generated sales of $59 million and $143 million resulting in royalty revenue of $13 million and $33 million in the third quarter and the nine months ended September 30, 2025 , respectively Launches underway in numerous regions, including the EU; additional submissions in progress to expand WAINUA access globally SPINRAZA® (nusinersen) for the treatment of spinal muscular atrophy (SMA) generated global sales of $374 million and $1.2 billion resulting in royalty revenue of $56 million and $158 million in the third quarter and the nine months ended September 30, 2025 , respectively
Launches underway in numerous regions, including the EU; additional submissions in progress to expand WAINUA access globally
Revenue
Ionis’ revenue was comprised of the following:
Three months ended
Nine months ended
September 30 ,
September 30 ,
2025
2024
2025
2024
Revenue:
(amounts in millions)
Commercial revenue:
Product sales, net:
TRYNGOLZA sales, net
$
32
$
-
$
57
$
-
Total product sales, net
32
-
57
-
Royalty revenue:
SPINRAZA royalties
56
57
158
152
WAINUA royalties
13
5
33
10
Other royalties
7
5
19
18
Total royalty revenue
76
67
210
180
Other commercial revenue
8
9
27
27
Total commercial revenue
116
76
294
207
Research and development revenue:
Collaborative agreement revenue
31
45
414
237
WAINUA joint development revenue
10
13
32
35
Total research and development revenue
41
58
446
272
Total revenue
$
157
$
134
$
740
$
479
Commercial revenue for the third quarter and the nine months ended September 30, 2025 , increased 53% and 42%, respectively, compared to the same periods in 2024. This increase was primarily driven by TRYNGOLZA product sales. Higher royalty revenue also contributed to the year over year increase.
The remainder of the Company’s revenue came from programs under its R&D collaborations, including a $280 million upfront payment for the global license of sapablursen to Ono Pharmaceutical Co., Ltd. in the second quarter of 2025, reflecting the value that Ionis’ pipeline and technology continues to generate.
Operating Expenses
SG&A expenses increased as anticipated for the third quarter and the nine months ended September 30, 2025 , compared to the same periods in 2024, primarily due to the launches of TRYNGOLZA, DAWNZERA and WAINUA. This increase was partially offset by a decrease in R&D expenses as several late-stage studies ended. Overall, this led to a modest year-over-year increase in total operating expenses, which was in line with expectations.
Balance Sheet
As of September 30, 2025 , Ionis’ cash, cash equivalents and short-term investments were $2.2 billion , compared to $2.3 billion on December 31, 2024 . Ionis’ working capital decreased over the same period primarily due to the reclassification of the Company’s 0% convertible notes as a current liability.
Webcast and Other Updates
Management will host a conference call and webcast to discuss Ionis’ third quarter 2025 results at 11:30 a.m. Eastern time on Wednesday, October 29, 2025 . Interested parties may access the webcast here. A webcast replay will be available for a limited time at the same address. To access the Company’s third quarter 2025 earnings slides click here.
Ionis’ Marketed Medicines
INDICATION for TRYNGOLZA® (olezarsen)
TRYNGOLZA® (olezarsen) was approved by the U.S. Food and Drug Administration as an adjunct to diet to reduce triglycerides in adults with familial chylomicronemia syndrome (FCS).
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
TRYNGOLZA is contraindicated in patients with a history of serious hypersensitivity to TRYNGOLZA or any of the excipients in TRYNGOLZA. Hypersensitivity reactions requiring medical treatment have occurred.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Hypersensitivity reactions (including symptoms of bronchospasm, diffuse erythema, facial swelling, urticaria, chills and myalgias) have been reported in patients treated with TRYNGOLZA. Advise patients on the signs and symptoms of hypersensitivity reactions and instruct patients to promptly seek medical attention and discontinue use of TRYNGOLZA if hypersensitivity reactions occur.
ADVERSE REACTIONS
The most common adverse reactions (incidence >5% of TRYNGOLZA-treated patients and >3% higher frequency than placebo) were injection site reactions, decreased platelet count and arthralgia.
Please see full Prescribing Information for TRYNGOLZA.
INDICATION for DAWNZERATM (donidalorsen)
DAWNZERA™ (donidalorsen) was approved by the U.S. Food and Drug Administration for prophylaxis to prevent attacks of hereditary angioedema ( HAE) in adult and pediatric patients 12 years of age and older.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
DAWNZERA is contraindicated in patients with a history of serious hypersensitivity reactions, including anaphylaxis, to donidalorsen or any of the excipients in DAWNZERA.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, have been reported in patients treated with DAWNZERA. If signs and symptoms of serious hypersensitivity reactions occur, discontinue DAWNZERA and institute appropriate therapy.
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 5%) are injection site reactions, upper respiratory tract infection, urinary tract infection, and abdominal discomfort.
Please see full Prescribing Information for DAWNZERA.
INDICATION for WAINUA® (eplontersen)
WAINUA injection, for subcutaneous use, 45 mg is indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults.
IMPORTANT SAFETY INFORMATION for WAINUA® (eplontersen)
WARNINGS AND PRECAUTIONS
Reduced Serum Vitamin A Levels and Recommended Supplementation WAINUA leads to a decrease in serum vitamin A levels. Supplement with recommended daily allowance of vitamin A. Refer patient to an ophthalmologist if ocular symptoms suggestive of vitamin A deficiency occur.
ADVERSE REACTIONS
Most common adverse reactions (≥9% in WAINUA-treated patients) were vitamin A decreased (15%) and vomiting (9%).
Please see link to U.S. Full Prescribing Information for WAINUA.
For more information about SPINRAZA and QALSODY, visit https://www.spinraza.com/ and https://www.qalsody.com/, respectively. QALSODY is approved under accelerated approval based on reduction in plasma neurofilament light chain (NfL) observed in patients treated with QALSODY. Continued approval may be contingent upon verification of clinical benefit in confirmatory trial(s).
About Ionis Pharmaceuticals, Inc.
For three decades, Ionis has invented medicines that bring better futures to people with serious diseases. Ionis currently has marketed medicines and a leading pipeline in neurology, cardiometabolic disease and select areas of high patient need. As the pioneer in RNA-targeted medicines, Ionis continues to drive innovation in RNA therapies in addition to advancing new approaches in gene editing. A deep understanding of disease biology and industry-leading technology propels our work, coupled with a passion and urgency to deliver life-changing advances for patients. To learn more about Ionis, visit Ionis.com and follow us on X (Twitter), LinkedIn and Instagram.
Ionis’ Forward-looking Statement
This press release includes forward-looking statements regarding Ionis’ business, financial guidance and the therapeutic and commercial potential of our commercial medicines, additional medicines in development, technologies and our expectations regarding development and regulatory milestones. Any statement describing Ionis’ goals, expectations, financial or other projections, intentions or beliefs is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to certain risks and uncertainties including those inherent in the process of discovering, developing and commercializing medicines that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such medicines. Ionis’ forward-looking statements also involve assumptions that, if they never materialize or prove correct, could cause its results to differ materially from those expressed or implied by such forward-looking statements. Although Ionis’ forward-looking statements reflect the good faith judgment of its management, these statements are based only on facts and factors currently known by Ionis. Except as required by law, we undertake no obligation to update any forward-looking statements for any reason. As a result, you are cautioned not to rely on these forward-looking statements. These and other risks concerning Ionis' programs are described in additional detail in Ionis' annual report on Form 10-K for the year ended December 31, 2024 , and most recent Form 10-Q, which are on file with the Securities and Exchange Commission. Copies of these and other documents are available from the Company.
In this press release, unless the context requires otherwise, “Ionis,” “Company,” “we,” “our” and “us” all refer to Ionis Pharmaceuticals and its subsidiaries.
IONIS® is a registered trademark of Ionis Pharmaceuticals, Inc. TRYNGOLZA® is a registered trademark of Ionis Pharmaceuticals, Inc. DAWNZERATM is a trademark of Ionis Pharmaceuticals, Inc. AKCEATM is a trademark of Akcea Therapeutics, Inc. TEGSEDITM is a trademark of Akcea Therapeutics, Inc. WAYLIVRATM is a trademark of Akcea Therapeutics, Inc. SPINRAZA® and QALSODY® are registered trademarks of Biogen. WAINUA® is a registered trademark of the AstraZeneca group of companies.
IONIS PHARMACEUTICALS, INC.
SELECTED FINANCIAL INFORMATION
Condensed Consolidated Statements of Operations
(In Millions, Except Per Share Data)
Three months ended
Nine months ended
September 30 ,
September 30 ,
2025
2024
2025
2024
(unaudited)
Revenue:
Commercial revenue:
Product sales, net
$
32
$
-
$
57
$
-
Royalty revenue
76
67
210
180
Other commercial revenue
8
9
27
27
Total commercial revenue
116
76
294
207
Research and development revenue:
Collaborative agreement revenue
31
45
414
237
WAINUA joint development revenue
10
13
32
35
Total research and development revenue
41
58
446
272
Total revenue
157
134
740
479
Expenses:
Cost of sales
2
1
8
7
Research, development and patent
218
220
636
656
Selling, general and administrative
97
61
263
180
Total operating expenses
317
282
907
843
Loss from operations
(160
)
(148
)
(167
)
(364
)
Other income (expense):
Interest expense related to the sale of future royalties
(18
)
(19
)
(55
)
(55
)
Other income, net
49
23
70
66
Loss before income tax benefit
(129
)
(144
)
(152
)
(353
)
Income tax benefit
-
4
-
3
Net loss
($
129
)
($
140
)
($
152
)
($
350
)
Basic and diluted net loss per share
($
0.80
)
($
0.95
)
($
0.95
)
($
2.38
)
Shares used in computing basic and diluted net loss per share
160
149
159
147
IONIS PHARMACEUTICALS, INC.
Reconciliation of GAAP to Non-GAAP Basis:
Condensed Consolidated Operating Expenses, Loss From Operations, and Net Loss
(In Millions)
Three months ended September 30 ,
Nine months ended September 30 ,
2025
2024
2025
2024
(unaudited)
As reported research, development and patent expenses according to GAAP
$
218
$
220
$
636
$
656
Excluding compensation expense related to equity awards
(21
)
(22
)
(61
)
(67
)
Non-GAAP research, development and patent expenses
$
197
$
198
$
575
$
589
As reported selling, general and administrative expenses according to GAAP
$
97
$
61
$
263
$
180
Excluding compensation expense related to equity awards
(10
)
(10
)
(29
)
(26
)
Non-GAAP selling, general and administrative expenses
$
87
$
51
$
234
$
154
As reported operating expenses according to GAAP
$
317
$
282
$
907
$
843
Excluding compensation expense related to equity awards
(31
)
(32
)
(91
)
(94
)
Non-GAAP operating expenses
$
286
$
250
$
816
$
749
As reported loss from operations according to GAAP
($
160
)
($
148
)
($
167
)
($
364
)
Excluding compensation expense related to equity awards
(31
)
(32
)
(91
)
(94
)
Non-GAAP loss from operations
($
129
)
($
116
)
($
76
)
($
270
)
As reported net loss according to GAAP
($
129
)
($
140
)
($
152
)
($
350
)
Excluding compensation expense related to equity awards and related tax effects
(31
)
(32
)
(91
)
(94
)
Non-GAAP net loss
($
98
)
($
108
)
($
61
)
($
256
)
Reconciliation of GAAP to Non-GAAP Basis
As illustrated in the Selected Financial Information in this press release, non-GAAP operating expenses, non-GAAP loss from operations, and non-GAAP net loss were adjusted from GAAP to exclude compensation expense related to equity awards and the related tax effects. Compensation expense related to equity awards are non-cash. These measures are provided as supplementary information and are not a substitute for financial measures calculated in accordance with GAAP. Ionis reports these non-GAAP results to better enable financial statement users to assess and compare its historical performance and project its future operating results and cash flows. Further, the presentation of Ionis’ non-GAAP results is consistent with how Ionis’ management internally evaluates the performance of its operations.
IONIS PHARMACEUTICALS, INC.
Condensed Consolidated Balance Sheets
(In Millions)
September 30 ,
December 31 ,
2025
2024
(unaudited)
Assets:
Cash, cash equivalents and short-term investments
$
2,240
$
2,298
Contracts receivable
25
92
Other current assets
254
230
Property, plant and equipment, net
106
94
Right-of-use assets
242
162
Other assets
166
127
Total assets
$
3,033
$
3,003
Liabilities and stockholders’ equity:
Current portion of deferred contract revenue
$
77
$
79
0% convertible senior notes, net – current
631
-
Other current liabilities
195
229
1.75% convertible senior notes, net
567
565
0% convertible senior notes, net
-
629
Liability related to sale of future royalties, net
545
542
Long-term lease liabilities
263
162
Long-term obligations, less current portion
29
52
Long-term deferred contract revenue
108
157
Total stockholders’ equity
618
588
Total liabilities and stockholders’ equity
$
3,033
$
3,003
Key 2025 and 2026 Value Driving Events(1)
New Product Launches
Program
Indication
2025
2026
DAWNZERA ( U.S. )
HAE
Achieved
TRYNGOLZA ( U.S. )
FCS
Achieved
WAINZUA (EU)
ATTRv-PN
Achieved
Olezarsen ( U.S. )
sHTG
•
Zilganersen ( U.S. )
Alexander disease
•
Regulatory Actions
Program
Indication
Regulatory Action
2025
2026
Donidalorsen
HAE
U.S. approval decision
Achieved
EU approval decision
•
TRYNGOLZA
FCS
EU approval decision
Achieved
Olezarsen
sHTG
U.S. submission
•
U.S. approval decision
•
Zilganersen
Alexander disease
U.S. submission
•
U.S. approval decision
•
Nusinersen
(higher dose)
SMA
U.S. and EU submissions
Achieved
U.S. approval decision
Refiling process
on track
WAINZUA
ATTRv-PN
EU approval decision
Achieved
Pelacarsen
Lp(a)- CVD
U.S. submission
•
Bepirovirsen
HBV
Regulatory submission(s)
•
Regulatory decision(s)
•
Key Phase 3 Clinical Events
Program
Indication
Event
2025
2026
Olezarsen
sHTG
CORE, CORE2 data
Achieved
Essence data
Achieved
Zilganersen
Alexander disease
Phase 3 data
Achieved
ION582
Angelman syndrome
Phase 3 study start
Achieved
Phase 3 enrollment completion
•
Pelacarsen
Lp(a)-CVD
Lp(a) HORIZON data
•
Bepirovirsen
HBV
B-Well data
•
Eplontersen
ATTR-CM
CARDIO-TTRansform data
•
Sefaxersen
IgAN
IMAGINATION data
•
Ulefnersen
FUS-ALS
FUSION data
•
Timing expectations based on current assumptions and subject to change.
Indicates that the milestone is anticipated in the respective year.
View source version on businesswire.com: https://www.businesswire.com/news/home/20251029676166/en/
Ionis Investor Contact: D. Wade Walke , Ph.D. IR@ionis.com 760-603-2331
Ionis Media Contact: Hayley Soffer media@ionis.com 760-603-4679
Source: Ionis Pharmaceuticals, Inc.
Drug ApprovalClinical ResultPhase 3Financial StatementBreakthrough Therapy
28 Oct 2025
GSK wants to focus a siRNA oligonuceleotide dubbed EMP-012 on patients with non-type 2 inflammation, which the pharma described as a “key patient sub-group where treatment options are limited.”\n GSK’s antibody-drug conjugate strategy may be making more headlines recently, but the British pharma hasn’t forgotten about oligonucleotides.The drugmaker is paying $85 million upfront to Empirico for the rights to a siRNA oligonuceleotide dubbed EMP-012 that’s already in a phase 1 study for chronic obstructive pulmonary disease (COPD). The candidate has “best-in-class” potential, according to GSK, which hopes to broaden EMP-012’s use to other inflammatory respiratory diseases.The oligonclueotide targets a specific inflammatory pathway that has “potential for a therapeutic approach that is agnostic of baseline type 2 inflammation, smoking or co-morbid disease,” GSK said in an Oct. 28 release.The aim is to focus the asset on patients with non-type 2 inflammation, which the pharma described as a “key patient sub-group where treatment options are limited.”As well as the upfront payment, GSK could ultimately pay out up to $660 million in development, regulatory and commercial milestone payments as well as tiered royalties on sales if EMP-012 makes it to market.GSK placed EMP-012 in the context of the pharma’s wider COPD efforts. As well as scoring an approval for Nucala in the respiratory condition this year, the drugmaker also picked up a PDE3/4 inhibitor from China’s Hengrui Pharma in July that the British company said at the time “supports GSK\'s ambition to treat patients across the widest spectrum of COPD.”Kaivan Khavandi, M.D., Ph.D., GSK’s global head of respiratory, immunology and inflammation R&D, said the agreement with Empirico “reflects our ambition to transform care in COPD by advancing novel targets, backed by data, to address underlying drivers of disease.”“With its expected long-acting characteristics and ability to target distinct inflammatory pathways, EMP-012 complements our pipeline of diverse modalities in COPD and builds on the current landscape of inhaled and biologic therapeutics in this area of substantial unmet need,” Khavandi added. Over the years, Empirico has penned collaborations with the likes of Ionis Pharmaceuticals and Abcellera. The San Diego-based biotech’s CEO Omri Gottesman said the deal with GSK “further validates the enormous potential of Empirico’s proprietary target discovery and siRNA platforms to rapidly generate differentiated clinical programs of significant value.”“GSK’s global reach and deep expertise in COPD will help to accelerate the development of EMP-012, and we look forward to working closely with GSK on advancing a potentially transformative precision medicine for patients with COPD and other inflammatory respiratory diseases,” Gottesman added.GSK is itself no stranger to oligonucleotides, short strands of synthetic DNA or RNA that can reduce, restore or modulate RNA through several different mechanisms. GSK’s oligonucleotide pipeline is led by bepirovirsen, a collaboration with Ionis Pharmaceuticals that is now in a pair of phase 3 trials as a potential “functional cure” for hepatitis B virus infection. The oligo portfolio also includes a collaboration with Arrowhead Pharmaceuticals for fatty liver disease, various assets acquired from Wave Life Sciences and a nonexclusive license to Elsie Biotechnologies’ platform.
OligonucleotidePhase 1Phase 3License out/in
30 Jul 2025
CARLSBAD, Calif.--(BUSINESS WIRE)--Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) (the “Company”) today reported financial results and provided key updates for the second quarter ended June 30, 2025.
“During the second quarter, we continued to build momentum across our business,” said Brett P. Monia, Ph.D., chief executive officer of Ionis. “Our strong performance included excellent commercial execution, resulting in a substantial increase in TRYNGOLZA revenues, our first independently launched medicine. We expect additional advancements in the second half, including Ionis’ second independent launch with donidalorsen for hereditary angioedema, anticipated next month, and important Phase 3 results for olezarsen in severe hypertriglyceridemia and zilganersen in Alexander disease. We believe these four programs collectively represent multi-billion-dollar revenue potential and a transformational opportunity for Ionis and for patients.”
Second Quarter 2025 Summary Financial Results(1):
Three months ended
Six months ended
June 30,
June 30,
2025
2024
2025
2024
(amounts in millions)
Total revenue
$452
$225
$584
$345
Operating expenses
$312
$291
$591
$560
Operating expenses on a non-GAAP basis
$282
$260
$532
$498
Income (loss) from operations
$140
($66)
($7)
($215)
Income (loss) from operations on a non-GAAP basis
$170
($35)
$52
($153)
(1)
Reconciliation of GAAP to non-GAAP basis contained later in this release.
Recent Financial Highlights
Revenue doubled in the second quarter of 2025 and increased nearly 70% in the first half compared to the same period last year, driven by the continued successful launch of TRYNGOLZA and increased royalty and R&D revenues
Operating expenses increased by single digits in the second quarter and first half of 2025, compared to the same periods last year, primarily due to investments related to commercialization efforts for TRYNGOLZA, donidalorsen and WAINUA
Increased 2025 financial guidance reflects an improved outlook for the full year, strong overall revenue performance experienced year-to-date, including the early strength in TRYNGOLZA revenues:
Full Year 2025 Guidance
Previous
Guidance
New
Guidance
Total Revenue
$725-750 million
$825-850 million
TRYNGOLZA product sales, net
Not provided
$75-80 million
Operating loss on a non-GAAP basis
<$375 million
$300-325 million
Cash, cash equivalents and short-term investments
~$1.9 billion
~$2.0 billion
Second Quarter 2025 Financial Results
“For the second time this year, we are significantly raising our 2025 financial guidance — this time driven by an improved outlook for the year and strong revenue performance to date, which includes the early launch excellence with TRYNGOLZA. In addition to strong commercial performance, our second quarter results included the substantial revenue we earned from licensing sapablursen, a medicine outside our core areas of focus. We are in a strong financial position, with a commitment to drive operating leverage as we continue executing on our strategic priorities,” said Elizabeth L. Hougen, chief financial officer, Ionis. “Moving forward, the three additional independent launches anticipated over the next eighteen months, including donidalorsen for hereditary angioedema, olezarsen in severe hypertriglyceridemia and zilganersen in Alexander disease, position Ionis to deliver substantial and growing product revenue. This product revenue, coupled with anticipated increasing royalty revenue from multiple partner launches, along with disciplined investment, position Ionis to achieve sustained growth and positive cash flow in the next few years.”
Recent Highlights - Wholly Owned Medicines
TRYNGOLZA TM (olezarsen), the first and only FDA approved treatment for adults living with familial chylomicronemia syndrome (FCS) as an adjunct to diet Generated net product sales of $19 million in the second quarter of 2025, its second full quarter on the market, and $26 million in the first half of 2025 Received a positive opinion from the Committee for Medicinal Products for Human Use (CHMP), paving the way to bring TRYNGOLZA to patients across Europe
Generated net product sales of $19 million in the second quarter of 2025, its second full quarter on the market, and $26 million in the first half of 2025
Received a positive opinion from the Committee for Medicinal Products for Human Use (CHMP), paving the way to bring TRYNGOLZA to patients across Europe
Olezarsen on track for topline Phase 3 data from pivotal CORE and CORE2 studies in patients with sHTG in September 2025, positioning olezarsen to potentially treat this second, more prevalent patient population with high unmet need Announced positive topline results from the Essence study in people with moderately elevated triglycerides; achieved primary and all key secondary endpoints for 80 mg and 50 mg monthly doses with favorable safety and tolerability
Announced positive topline results from the Essence study in people with moderately elevated triglycerides; achieved primary and all key secondary endpoints for 80 mg and 50 mg monthly doses with favorable safety and tolerability
Donidalorsen on track to launch this year, assuming approval, with a U.S. PDUFA date of August 21, 2025 Poised to transform the treatment paradigm for individuals with hereditary angioedema (HAE) as the first and only RNA-targeted prophylactic therapy that has the potential to offer durable efficacy, a favorable safety and tolerability profile, and the longest available dosing interval, with self-administration via autoinjector monthly or every other month Donidalorsen is currently under regulatory review in the EU
Poised to transform the treatment paradigm for individuals with hereditary angioedema (HAE) as the first and only RNA-targeted prophylactic therapy that has the potential to offer durable efficacy, a favorable safety and tolerability profile, and the longest available dosing interval, with self-administration via autoinjector monthly or every other month
Donidalorsen is currently under regulatory review in the EU
First patient dosed in the Phase 3 REVEAL study of ION582, an investigational medicine for the treatment of people living with Angelman syndrome (AS), a serious and rare neurodevelopmental disorder
Recent Highlights – Partnered Medicines
WAINUA TM (eplontersen) (WAINZUA in EU) for the treatment of adults with polyneuropathy of hereditary transthyretin-mediated amyloidosis (ATTRv-PN) continues to perform well, achieving several important commercial milestones: Generated sales of $44 million and $84 million resulting in royalty revenue of $10 million and $20 million in the second quarter and first half of 2025, respectively New launches underway in numerous regions, including the EU; additional submissions in progress to expand WAINUA access globally
Generated sales of $44 million and $84 million resulting in royalty revenue of $10 million and $20 million in the second quarter and first half of 2025, respectively
New launches underway in numerous regions, including the EU; additional submissions in progress to expand WAINUA access globally
SPINRAZA ® (nusinersen) for the treatment of spinal muscular atrophy (SMA) generated global sales of $393 million and $817 million resulting in royalty revenue of $54 million and $102 million in the second quarter and first half of 2025, respectively Higher dose nusinersen under review for marketing approval in the U.S. (PDUFA date of September 22, 2025) and in the EU
Higher dose nusinersen under review for marketing approval in the U.S. (PDUFA date of September 22, 2025) and in the EU
Biogen to advance salanersen (formerly ION306/BIIB115), an investigational medicine for SMA into registrational studies based on positive interim Phase 1 results; developed using novel Ionis antisense chemistry with the potential to achieve high efficacy and annual dosing Phase 1 data with salanersen in SMA patients showed substantial slowing of neurodegeneration and clinically meaningful improvements in patients previously treated with gene therapy
Phase 1 data with salanersen in SMA patients showed substantial slowing of neurodegeneration and clinically meaningful improvements in patients previously treated with gene therapy
AstraZeneca initiated the Phase 2b study of opemalirsen (formerly ION532/AZD2373), an investigational medicine designed to reduce the production of apolipoprotein L1 (APOL1) for the treatment of APOL1-mediated kidney disease (AMKD) triggering a $30 million milestone payment to Ionis
Corporate Updates
In June 2025, Ionis announced that Richard Geary, Ph.D., executive vice president and chief development officer, will retire effective January 2026 and that Holly Kordasiewicz, Ph.D., currently senior vice president, neurology, will succeed him in the role
Revenue
Ionis’ revenue was comprised of the following:
Three months ended
Six months ended
June 30,
June 30,
2025
2024
2025
2024
Revenue:
(amounts in millions)
Commercial revenue:
Product sales, net:
TRYNGOLZA sales, net
$19
$-
$26
$-
Total product sales, net
19
-
26
-
Royalty revenue:
SPINRAZA royalties
54
57
102
95
WAINUA royalties
10
4
20
5
Other royalties
6
3
12
13
Total royalty revenue
70
64
134
113
Other commercial revenue:
TEGSEDI and WAYLIVRA revenue, net
14
8
19
17
Other revenue
-
-
-
2
Total commercial revenue
103
72
179
132
Research and development revenue:
Collaborative agreement revenue
337
141
382
191
WAINUA joint development revenue
12
12
23
22
Total research and development revenue
349
153
405
213
Total revenue
$452
$225
$584
$345
Commercial revenue for the second quarter and first half of 2025 increased 43% and 36% respectively, compared to the same periods in 2024. This increase was driven by TRYNGOLZA product sales. Higher royalty revenue also contributed to the year over year increase.
The remainder of the Company’s revenue came from programs under its R&D collaborations, including a $280 million upfront payment for the global license of sapablursen to Ono Pharmaceutical Co., Ltd., reflecting the value that Ionis’ pipeline and technology continues to generate.
Operating Expenses
SG&A expenses increased as anticipated for the second quarter and first half of 2025, compared to the same periods in 2024, primarily due to the launches of TRYNGOLZA and WAINUA, and advancing launch preparation activities for donidalorsen. This increase was partially offset by a decrease in R&D expenses as several late-stage studies ended. Overall, this led to a modest year-over-year increase in total operating expenses.
Balance Sheet
As of June 30, 2025, Ionis’ cash, cash equivalents and short-term investments were $2.3 billion, consistent with December 31, 2024. Ionis received $280 million from the global license of sapablursen in the second quarter of 2025. Ionis’ working capital decreased over the same period primarily due to the reclassification of the Company’s 0% convertible notes as a current liability.
Webcast and Other Updates
Management will host a conference call and webcast to discuss Ionis’ second quarter 2025 results at 11:30 a.m. Eastern time on Wednesday, July 30, 2025. Interested parties may access the webcast here. A webcast replay will be available for a limited time at the same address. To access the Company’s second quarter 2025 earnings slides click here.
Ionis will be initiating a quiet period starting July 31, 2025, as the Company plans to announce the topline results from both the CORE and CORE2 studies simultaneously. The quiet period will be lifted upon the data announcement, expected in September.
Ionis’ Marketed Medicines
INDICATION for TRYNGOLZA™ (olezarsen)
TRYNGOLZA™ (olezarsen) was approved by the U.S. Food and Drug Administration as an adjunct to diet to reduce triglycerides in adults with familial chylomicronemia syndrome (FCS).
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
TRYNGOLZA is contraindicated in patients with a history of serious hypersensitivity to TRYNGOLZA or any of the excipients in TRYNGOLZA. Hypersensitivity reactions requiring medical treatment have occurred.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Hypersensitivity reactions (including symptoms of bronchospasm, diffuse erythema, facial swelling, urticaria, chills and myalgias) have been reported in patients treated with TRYNGOLZA. Advise patients on the signs and symptoms of hypersensitivity reactions and instruct patients to promptly seek medical attention and discontinue use of TRYNGOLZA if hypersensitivity reactions occur.
ADVERSE REACTIONS
The most common adverse reactions (incidence >5% of TRYNGOLZA-treated patients and >3% higher frequency than placebo) were injection site reactions, decreased platelet count and arthralgia.
Please see full Prescribing Information for TRYNGOLZA.
INDICATION for WAINUA™ (eplontersen)
WAINUA injection, for subcutaneous use, 45 mg is indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults.
IMPORTANT SAFETY INFORMATION for WAINUA™ (eplontersen)
WARNINGS AND PRECAUTIONS
Reduced Serum Vitamin A Levels and Recommended Supplementation WAINUA leads to a decrease in serum vitamin A levels. Supplement with recommended daily allowance of vitamin A. Refer patient to an ophthalmologist if ocular symptoms suggestive of vitamin A deficiency occur.
ADVERSE REACTIONS
Most common adverse reactions (≥9% in WAINUA-treated patients) were vitamin A decreased (15%) and vomiting (9%).
Please see link to U.S. Full Prescribing Information for WAINUA.
For more information about SPINRAZA and QALSODY, visit https://www.spinraza.com/ and https://www.qalsody.com/, respectively. QALSODY is approved under accelerated approval based on reduction in plasma neurofilament light chain (NfL) observed in patients treated with QALSODY. Continued approval may be contingent upon verification of clinical benefit in confirmatory trial(s).
About Ionis Pharmaceuticals, Inc.
For three decades, Ionis has invented medicines that bring better futures to people with serious diseases. Ionis currently has marketed medicines and a leading pipeline in neurology, cardiology, and other areas of high patient need. As the pioneer in RNA-targeted medicines, Ionis continues to drive innovation in RNA therapies in addition to advancing new approaches in gene editing. A deep understanding of disease biology and industry-leading technology propels our work, coupled with a passion and urgency to deliver life-changing advances for patients. To learn more about Ionis, visit Ionis.com and follow us on X (Twitter), LinkedIn and Instagram.
Ionis’ Forward-looking Statement
This press release includes forward-looking statements regarding Ionis’ business, financial guidance and the therapeutic and commercial potential of our commercial medicines, additional medicines in development and technologies. Any statement describing Ionis’ goals, expectations, financial or other projections, intentions or beliefs is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to certain risks and uncertainties including those inherent in the process of discovering, developing and commercializing medicines that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such medicines. Ionis’ forward-looking statements also involve assumptions that, if they never materialize or prove correct, could cause its results to differ materially from those expressed or implied by such forward-looking statements. Although Ionis’ forward-looking statements reflect the good faith judgment of its management, these statements are based only on facts and factors currently known by Ionis. Except as required by law, we undertake no obligation to update any forward-looking statements for any reason. As a result, you are cautioned not to rely on these forward-looking statements. These and other risks concerning Ionis' programs are described in additional detail in Ionis' annual report on Form 10-K for the year ended December 31, 2024, and most recent Form 10-Q, which are on file with the Securities and Exchange Commission. Copies of these and other documents are available from the Company.
In this press release, unless the context requires otherwise, “Ionis,” “Company,” “we,” “our” and “us” all refer to Ionis Pharmaceuticals and its subsidiaries.
IONIS® is a registered trademark of Ionis Pharmaceuticals, Inc. TRYNGOLZA® is a registered trademark of Ionis Pharmaceuticals, Inc. AKCEATM is a trademark of Akcea Therapeutics, Inc. TEGSEDITM is a trademark of Akcea Therapeutics, Inc. WAYLIVRATM is a trademark of Akcea Therapeutics, Inc. SPINRAZA® and QALSODY® are registered trademarks of Biogen. WAINUA® is a registered trademark of the AstraZeneca group of companies.
IONIS PHARMACEUTICALS, INC.
SELECTED FINANCIAL INFORMATION
Condensed Consolidated Statements of Operations
(In Millions, Except Per Share Data)
Three months ended
Six months ended
June 30,
June 30,
2025
2024
2025
2024
(unaudited)
Revenue:
Commercial revenue:
Product sales, net
$19
$-
$26
$-
Royalty revenue
70
64
134
113
Other commercial revenue
14
8
19
19
Total commercial revenue
103
72
179
132
Research and development revenue:
Collaborative agreement revenue
337
141
382
191
WAINUA joint development revenue
12
12
23
22
Total research and development revenue
349
153
405
213
Total revenue
452
225
584
345
Expenses:
Cost of sales
4
4
6
6
Research, development and patent
217
222
418
436
Selling, general and administrative
91
65
167
118
Total operating expenses
312
291
591
560
Income (loss) from operations
140
(66)
(7)
(215)
Other income (expense):
Interest expense related to the sale of future royalties
(19)
(18)
(37)
(36)
Other income, net
3
18
21
42
Income (loss) before income tax benefit (expense)
124
(66)
(23)
(209)
Income tax benefit (expense)
-
-
-
-
Net income (loss)
$124
($66)
($23)
($209)
Basic net income (loss) per share
$0.78
($0.45)
($0.15)
($1.43)
Diluted net income (loss) per share
$0.70
($0.45)
($0.15)
($1.43)
Shares used in computing basic net income (loss) per share
159
146
159
146
Shares used in computing diluted net income (loss) per share
182
146
159
146
IONIS PHARMACEUTICALS, INC.
Reconciliation of GAAP to Non-GAAP Basis:
Condensed Consolidated Operating Expenses, Income (Loss) From Operations, and Net Income (Loss)
(In Millions)
Three months ended
June 30,
Six months ended
June 30,
2025
2024
2025
2024
(unaudited)
As reported research, development and patent expenses according to GAAP
$217
$222
$418
$436
Excluding compensation expense related to equity awards
(20)
(23)
(40)
(45)
Non-GAAP research, development and patent expenses
$197
$199
$378
$391
As reported selling, general and administrative expenses according to GAAP
$91
$65
$167
$118
Excluding compensation expense related to equity awards
(10)
(8)
(19)
(17)
Non-GAAP selling, general and administrative expenses
$81
$57
$148
$101
As reported operating expenses according to GAAP
$312
$291
$591
$560
Excluding compensation expense related to equity awards
(30)
(31)
(59)
(62)
Non-GAAP operating expenses
$282
$260
$532
$498
As reported income (loss) from operations according to GAAP
$140
($66)
($7)
($215)
Excluding compensation expense related to equity awards
(30)
(31)
(59)
(62)
Non-GAAP income (loss) from operations
$170
($35)
$52
($153)
As reported net income (loss) according to GAAP
$124
($66)
($23)
($209)
Excluding compensation expense related to equity awards and related tax effects
(30)
(31)
(59)
(62)
Non-GAAP net income (loss)
$154
($35)
$36
($147)
Reconciliation of GAAP to Non-GAAP Basis
As illustrated in the Selected Financial Information in this press release, non-GAAP operating expenses, non-GAAP income (loss) from operations, and non-GAAP net income (loss) were adjusted from GAAP to exclude compensation expense related to equity awards and the related tax effects. Compensation expense related to equity awards are non-cash. These measures are provided as supplementary information and are not a substitute for financial measures calculated in accordance with GAAP. Ionis reports these non-GAAP results to better enable financial statement users to assess and compare its historical performance and project its future operating results and cash flows. Further, the presentation of Ionis’ non-GAAP results is consistent with how Ionis’ management internally evaluates the performance of its operations.
IONIS PHARMACEUTICALS, INC.
Condensed Consolidated Balance Sheets
(In Millions)
June 30,
December 31,
2025
2024
(unaudited)
Assets:
Cash, cash equivalents and short-term investments
$2,290
$2,298
Contracts receivable
53
92
Other current assets
235
230
Property, plant and equipment, net
112
94
Right-of-use assets
164
162
Other assets
131
127
Total assets
$2,985
$3,003
Liabilities and stockholders’ equity:
Current portion of deferred contract revenue
$76
$79
0% convertible senior notes, net – current
630
-
Other current liabilities
191
229
1.75% convertible senior notes, net
566
565
0% convertible senior notes, net
-
629
Liability related to sale of future royalties, net
541
542
Long-term lease liabilities
164
162
Long-term obligations, less current portion
60
52
Long-term deferred contract revenue
125
157
Total stockholders’ equity
632
588
Total liabilities and stockholders’ equity
$2,985
$3,003
Key 2025 and 2026 Value Driving Events(1)
New Product Launches
Program
Indication
2025
2026
Donidalorsen (U.S.)
HAE
•
TRYNGOLZA (U.S.)
FCS
Achieved
WAINZUA (EU)
ATTRv-PN
Achieved
Olezarsen (U.S.)
sHTG
•
Zilganersen (U.S.)
Alexander disease
•
Regulatory Actions
Program
Indication
Regulatory Action
2025
2026
Donidalorsen
HAE
U.S. approval decision
•
EU approval decision
•
TRYNGOLZA
FCS
EU approval decision
•
Olezarsen
sHTG
U.S. submission
•
U.S. approval decision
•
Zilganersen
Alexander disease
U.S. submission
•
U.S. approval decision
•
Nusinersen
(higher dose)
SMA
U.S. and EU submissions
Achieved
U.S. approval decision
•
WAINZUA
ATTRv-PN
EU approval decision
Achieved
Pelacarsen
Lp(a)- CVD
U.S. submission
•
Bepirovirsen
HBV
Regulatory submission(s)
•
Regulatory decision(s)
•
Key Phase 3 Clinical Events
Program
Indication
Event
2025
2026
Olezarsen
sHTG
CORE, CORE2 data
•
Essence data
Achieved
Zilganersen
Alexander disease
Phase 3 data
•
ION582
Angelman syndrome
Phase 3 study start
Achieved
Phase 3 enrollment completion
•
Pelacarsen
Lp(a)-CVD
Lp(a) HORIZON data
•
Bepirovirsen
HBV
B-Well data
•
Eplontersen
ATTR-CM
CARDIO-TTRansform data
•
Sefaxersen
IgAN
IMAGINATION data
•
Ulefnersen
FUS-ALS
FUSION data
•
(1)
Timing expectations based on current assumptions and subject to change.
Indicates that the milestone is anticipated in the respective year.
Clinical ResultPhase 1Drug ApprovalPhase 3Phase 2
100 Deals associated with Bepirovirsen
Login to view more data
External Link
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| - | - | - |
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Hepatitis B, Chronic | Phase 3 | United States | 06 Dec 2022 | |
| Hepatitis B, Chronic | Phase 3 | China | 06 Dec 2022 | |
| Hepatitis B, Chronic | Phase 3 | Japan | 06 Dec 2022 | |
| Hepatitis B, Chronic | Phase 3 | Argentina | 06 Dec 2022 | |
| Hepatitis B, Chronic | Phase 3 | Australia | 06 Dec 2022 | |
| Hepatitis B, Chronic | Phase 3 | Brazil | 06 Dec 2022 | |
| Hepatitis B, Chronic | Phase 3 | Bulgaria | 06 Dec 2022 | |
| Hepatitis B, Chronic | Phase 3 | Canada | 06 Dec 2022 | |
| Hepatitis B, Chronic | Phase 3 | France | 06 Dec 2022 | |
| Hepatitis B, Chronic | Phase 3 | Germany | 06 Dec 2022 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 1 | 160 | (Bepirovirsen 300 mg Vial by HCP) | vbiitzoqog(eudscpnyex) = qsbkmjaaus whorjhvvvs (eedhixatps, 0.049) View more | - | 18 Jul 2025 | ||
(Bepirovirsen 300 mg PFS SSD by HCP) | vbiitzoqog(eudscpnyex) = kaorgrddrk whorjhvvvs (eedhixatps, 0.048) View more | ||||||
Phase 2 | Hepatitis B, Chronic HBsAg | HBV DNA | 108 | ihahordvtm(chtbtexult) = The proportions of participants with adverse events and treatment-related adverse events in both treatment windows were similar between treatment arms svimsakakf (zsoipumfzd ) | Positive | 01 Feb 2025 | ||
Phase 2 | 12 | Nucleos(t)ide therapy+GSK3228836 | hoobrrwfqr = zhpjtzfsyq yyveoahirk (zyfqskfdyk, lksddchckr - zttcpoelzt) View more | - | 19 Dec 2024 | ||
Phase 1 | 24 | (hepatic impairment participants) | jeyschyurm(ommyaybzmh): Geometric Mean Ratio = 0.69 View more | Positive | 13 Sep 2024 | ||
(healthy participants) | |||||||
Phase 2 | 108 | PegIFN+GSK3228836 (GSK3228836 300 mg (24 Weeks) + PegIFN 180 mcg (24 Weeks)) | rwhmolkrij = pdpfyklqhn hjschejkkx (opaucnyifn, lakvyingkw - kbjvykleyq) View more | - | 02 May 2024 | ||
PegIFN+GSK3228836 (GSK3228836 300 mg (12 Weeks) + PegIFN 180 mcg (24 Weeks)) | qwpqidddxq = puawezubns ocbywchgit (toakijqidj, tnxxkjbppt - pyilkuairr) View more | ||||||
Phase 2 | Hepatitis B, Chronic HBsAg | HBV DNA | ALT | 108 | Arm 1 (BPV 300 mg for 24 weeks + PegIFN 180 mcg QW for 24 weeks) | tlrsbevnfz(avivrhqaal) = vhrnwladiz xuvlfappkj (ibubmkipcn ) | - | 10 Nov 2023 | |
Phase 2 | - | Arm 1 (BPV 24 wk) | tzgqwleelh(vuqqbdqrxg) = sssfwgqotg imfzgmdibb (yjsndnpefp ) | - | 10 Nov 2023 | ||
Arm 2 (BPV 12 wk) | tzgqwleelh(vuqqbdqrxg) = wkqerewidw imfzgmdibb (yjsndnpefp ) |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free

