Verrica notches long-awaited FDA nod but shares plummet after loan revelation
24 Jul 2023
Phase 3Drug ApprovalClinical Result
Preview
Source: FiercePharma
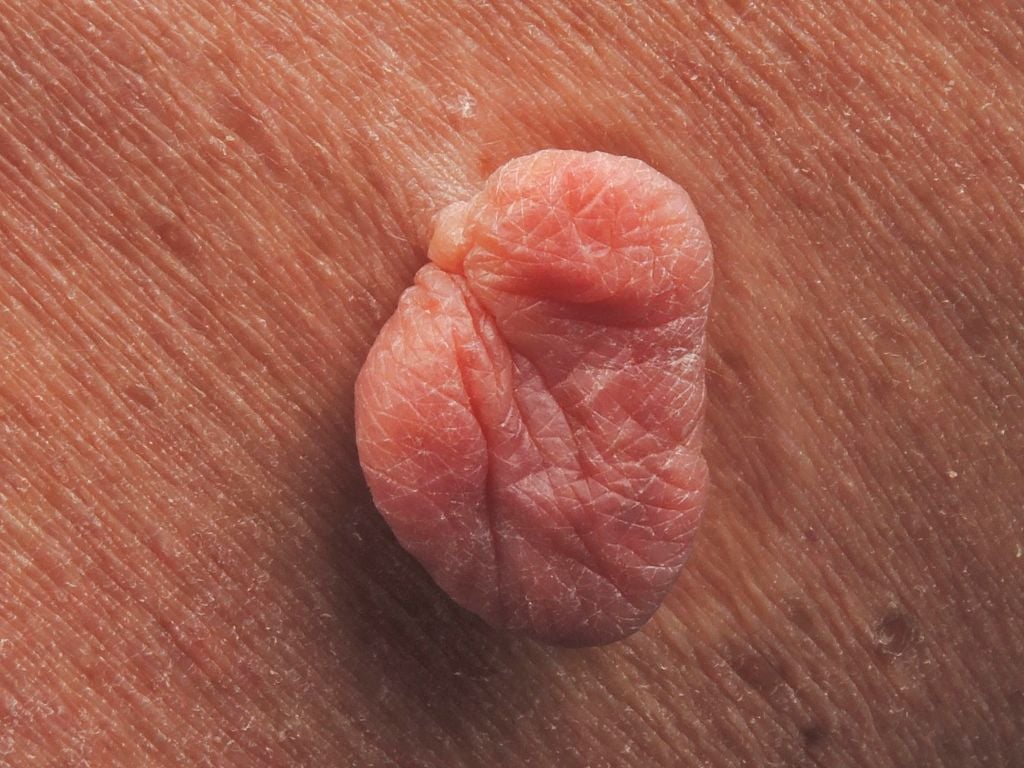
Verrica's Ycanth has been approved by the FDA, becoming the first treatment for molluscum, an under-diagnosed skin condition that strikes roughly 6 million in the U.S. each year.
After two rejections from the FDA—both related to manufacturing—Verrica Pharmaceuticals has scored approval for Ycanth (cantharidin/VP-102), which becomes the first therapeutic sanctioned in the United States for molluscum, a viral skin infection.
On Monday, after the Friday thumbs up from the FDA, Verrica said it was entering a non-binding term sheet for up to $125 million in debt financing to support the launch, which will come in late September.
When Verrica closes the loan deal, which it expects to do by the end of this week, the company will borrow $50 million immediately, it said. That, in addition to the $60 million in cash and equivalents it had on hand at the end of the first quarter, extends the company’s runway to the first quarter of 2025, Verrica said.
Hours after Monday’s revelation of the loan, Verrica’s shares had dipped by 30% from close on Friday.
This is the first approval for the 10-year-old company which is based in West Chester, Pa. and specializes in dermatology therapeutics for skin diseases. The company also is investigating cantharidin as a treatment for genital warts and common warts.
Molluscum is an under-diagnosed contagious condition that strikes roughly 6 million annually in the U.S., most of them children. The approval of the topical treatment covers those ages 2 and older.
Molluscum is spread by skin-to-skin contact or by contact with objects that can carry the virus such as toys, towels or other wet surfaces. It causes raised lesions that can produce inflammation, itching and bacterial infection. The lesions can last for years and sometimes produce permanent scarring.
The company estimates that only 15% of molluscum cases are diagnosed but CEO Ted White said in a conference call on Monday that he expects more diagnoses now that a treatment has been endorsed. The company projects the market to be 1 million patients per year.
“Right now, we’re staffed to capture about 85% of the market,” Joe Bonaccorso, the company’s chief commercial officer, said on the call.
The company has yet to arrive at a price for Ycanth. That should come sometime next month, added CFO Terry Kohler.
The approval was based on two phase 3 trials, which enrolled 518 patients and showed that VP-102 produced 50% clearance of lesions versus 15% for those on a placebo treatment. Results remained consistent when comparing age groups and location of lesions.
The approval was a long time coming after Verrica was hit with complete response letters (CRLs) in September 2021 and May 2022.
In between the two rejections, Verrica’s contract manufacturer Sterling Pharmaceuticals Services was placed on official action indicated (OAI) status, which is the most serious category of violation. After the second CRL, Verrica said that—in addition to working with Sterling to remedy the problems—that it was also engaging another CMO to supply the drug.
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Indications
Targets
-Drugs
Chat with Hiro
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.




