CRISPR Therapeutics Announces Updates to Immuno-Oncology Pipeline and Expansion into Autoimmune Disease
06 Dec 2023
ImmunotherapyClinical StudyGene Therapy
-Based on preliminary data from ongoing clinical trials, focusing on next-generation CAR T product candidates, CTX112™ targeting CD19 and CTX131™ targeting CD70-
-Expanding trials of CTX112 into autoimmune disease, with planned initiation in the first half of 2024, in addition to the ongoing clinical trial in B-cell malignancies-
-Expanding trials of CTX131 into hematologic malignancies, including T- and B-cell malignancies, in addition to the ongoing clinical trial in solid tumors-
-Next-generation candidates manufactured at internal GMP facility exhibit increased manufacturing robustness and scalability-
CRISPR Therapeutics (Nasdaq: CRSP), a biopharmaceutical company focused on creating transformative gene-based medicines for serious diseases, today provided an update on its immuno-oncology pipeline of CRISPR/Cas9 gene-edited allogeneic chimeric antigen receptor (CAR) T cell product candidates.
The Company’s first-generation allogeneic CAR T candidates, CTX110 and CTX130, provided important proof of concept that allogeneic CAR T cells can produce durable remissions following a standard lymphodepletion regimen. Preliminary data from ongoing clinical trials of its next-generation candidates, CTX112 targeting CD19 and CTX131 targeting CD70, suggest that these candidates may improve upon that clinical profile. Emerging pharmacology data, including pharmacokinetics, indicate that the novel potency gene edits in CTX112 and CTX131 lead to significantly higher CAR T cell expansion and functional persistence in patients compared to the first-generation candidates. In addition, the next-generation candidates exhibit increased manufacturing robustness, with a higher and more consistent number of CAR T cells produced per batch. Based on these considerations, the Company is focusing on the development of CTX112 and CTX131 and will be transitioning patients treated with CTX110 and CTX130 to long-term follow-up where applicable.
“Our next-generation allogeneic CAR T candidates reflect our mission of innovating continuously to bring potentially transformative medicines to patients as quickly as possible,” said Samarth Kulkarni, Ph.D., Chief Executive Officer and Chairman of the Board of CRISPR Therapeutics. “We are excited about our next-generation CAR T platform, and focusing our efforts on these candidates will allow us to advance these potentially best-in-class CAR T therapies more efficiently and rapidly.”
“We are very encouraged by the progress and early clinical data from our next-generation candidates. While we saw benefits from consolidation dosing with CTX110, we believe CTX112 could result in even better outcomes for patients,” said PK Morrow, M.D., Chief Medical Officer of CRISPR Therapeutics. “We thank the patients, families, and investigators who have participated in our clinical trials of CTX110 and CTX130 and look forward to applying learnings from these programs to expedite the development of CTX112 and CTX131.”
In December 2022, the Company presented data from Part A of the Phase 1/2 clinical trial of CTX110 that showed the potential for CTX110 to produce durable complete remissions in heavily pre-treated patients following a standard lymphodepletion regimen. In new data updated today, Part B of the trial demonstrated an increased 6-month complete response (CR) rate following the inclusion of consolidation dosing, as shown in the table below. The safety profile of CTX110 in Part B remained consistent with the positively differentiated safety profile observed in Part A.
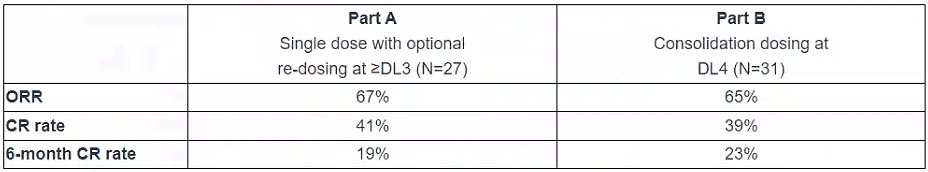
Preview
Source: GlobeNewswire-Health Care
CTX112 and CTX131 have the potential to improve upon the efficacy observed with CTX110 and CTX130. These next-generation candidates each incorporate two novel gene edits—knock-out of Regnase-1 and transforming growth factor-beta receptor type 2 (TGFBR2)—that have the potential to enhance CAR T potency and reduce CAR T exhaustion. Editing Regnase-1 removes an intrinsic “brake” on T cell function while editing TGFBR2 removes a key extrinsic “brake” on T cell anti-tumor activity. CRISPR Therapeutics identified this combination of edits through systematic screening of dozens of new and previously described genes. In preclinical studies, these edits synergistically improved potency approximately 10-fold compared to the first-generation candidates. Clinical trials are ongoing for CTX112 in B-cell malignancies and for CTX131 in solid tumors. The Company is producing CTX112 and CTX131 for clinical trials at its internal GMP manufacturing facility.
Furthermore, CRISPR Therapeutics announced plans to initiate new clinical trials of CTX112 and CTX131 in additional indications. The Company plans to expand the evaluation of CTX112 beyond oncology into autoimmune diseases. Early clinical studies have shown that CD19-directed autologous CAR T therapy can produce long-lasting remissions in multiple autoimmune indications. CTX112 has the potential to provide similar results with several advantages, including greater scalability, lower cost of goods, and no patient apheresis. The Company plans to initiate a clinical trial in systemic lupus erythematosus (SLE) in the first half of 2024, with the potential to expand into additional autoimmune indications in the future.
About CD19 Candidates
CTX110 is a wholly-owned, healthy donor-derived gene-edited allogeneic CAR T investigational therapy targeting cluster of differentiation 19, or CD19, and CTX112 is a next-generation, wholly-owned, allogeneic CAR T product candidate targeting CD19, which incorporates additional edits designed to enhance CAR T potency and reduce CAR T exhaustion. CTX112 is being investigated in an ongoing clinical trial designed to assess safety and efficacy of the product candidate in adult patients with relapsed or refractory CD19-positive B-cell malignancies who have received at least two prior lines of therapy.
About CD70 Candidates
CTX130 is a wholly-owned, healthy donor-derived gene-edited allogeneic CAR T investigational therapy targeting cluster of differentiation 70, or CD70, an antigen expressed on various solid tumors and hematologic malignancies, and CTX131 is a next-generation, wholly-owned, allogeneic CAR T product candidate targeting CD70, which incorporates additional edits designed to enhance CAR T potency and reduce CAR T exhaustion. CTX131 is being investigated in a clinical trial designed to assess the safety and efficacy of the product candidate in adult patients with relapsed or refractory solid tumors.
About CRISPR Therapeutics
CRISPR Therapeutics is a leading gene editing company focused on developing transformative gene-based medicines for serious diseases using its proprietary CRISPR/Cas9 platform. CRISPR/Cas9 is a revolutionary gene editing technology that allows for precise, directed changes to genomic DNA. CRISPR Therapeutics has established a portfolio of therapeutic programs across a broad range of disease areas including hemoglobinopathies, oncology, regenerative medicine and cardiometabolic diseases. To accelerate and expand its efforts, CRISPR Therapeutics has established strategic partnerships with leading companies including Bayer, Vertex Pharmaceuticals and ViaCyte, Inc. CRISPR Therapeutics AG is headquartered in Zug, Switzerland, with its wholly-owned U.S. subsidiary, CRISPR Therapeutics, Inc., and R&D operations based in Boston, Massachusetts and San Francisco, California, and business offices in London, United Kingdom. For more information, please visit www.crisprtx.com.
CRISPR THERAPEUTICS® standard character mark and design logo, CTX110®, CTX112™, CTX130™, and CTX131™ are trademarks and registered trademarks of CRISPR Therapeutics AG. All other trademarks and registered trademarks are the property of their respective owners.
The content above comes from the network. if any infringement, please contact us to modify.
Organizations
Indications
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.





