APAC leaps ahead in adoptive cell therapy space
17 Jun 2024
Cell TherapyImmunotherapyClinical Study
Preview
Source: Pharmaceutical Technology
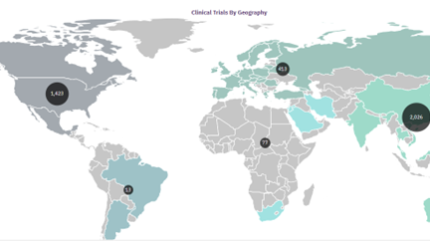
Caption: Credit: Scotty Chung-Siu via GlobalData. The APAC region is the leading area for the clinical development of adoptive cell therapies.

Preview
Source: Pharmaceutical Technology
The Asia Pacific (APAC) region is leading the charge for adoptive cell therapy studies, says Scotty Chung-Siu, a managing analyst at GlobalData.
At the Annual Outsourcing in Clinical Trials conference, held from 11-12 June, Chung-Siu led a comprehensive session on adoptive cell therapies, giving an overview of the space to the audience of clinical trial professionals.
Chung Siu cited a GlobalData report that demonstrated that the number of ongoing single-country adoptive cell therapy studies in the APAC region has reached approximately twelve times the number of trials in Europe. He also highlighted that overall, there are 1,023 single country adoptive cell therapy clinical studies being run in the APAC region compared to 476 in North America.
GlobalData is the parent company of Pharmaceutical Technology.
Adoptive cell therapies are immunotherapies that use engineered cells to trigger a patient’s immune system to treat a condition. The three main types of adoptive cell therapies are tumour-infiltrating lymphocyte (TIL) immunotherapy, natural killer (NK) cell immunotherapy, and T cell immunotherapies. The last category includes several modalities such as chimeric antigen T (CAR-T) cell therapies, natural killer T cells, regulatory T cells, and T cell receptor (TCR) therapies.
See Also:AZD-1705 by AstraZeneca for Dyslipidemia: Likelihood of Approval

Preview
Source: Pharmaceutical Technology
Sabestomig by AstraZeneca for Crohn’s Disease (Regional Enteritis): Likelihood of Approval

Preview
Source: Pharmaceutical Technology
Blockbuster adoptive cell therapies such as Novartis’ CAR-T therapy Kymriah (tisagenlecleucel), and Gilead Sciences’ and Daiichi Sankyo’s Yescarta (axicabtagene ciloleucel) are commercially available in some Asian countries, with the former company placing cell therapy manufacturing sites in Japan in 2020.
A February 2024 paper published in the Blood Cell Therapy journal, reported that Asia has driven 70% of global economic growth in the last two years, and estimated a compound annual growth rate for CAR-T adoption in Asia of 40%.
Chung Siu also pointed to the Bongguan, Hebei, China-headquartered Hebei Senlang Biotechnology and the Chongqing, China-based Chongqin Precision Biotech as some of the top industry sponsors, leading with 34 and 32 adoptive cell therapy trials, respectively.
The London conference held sessions discussing a wide range of topics in the field of clinical research, ranging from the UK clinical trial landscape to the use of AI in clinical trials.
Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva. Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.
Free WhitepaperOptimise your cell therapy process: a guide to cell thawing
Typically carried out at the point of care, errors in cell therapy thawing could compromise treatment efficacy, leading to significant patient impact as well as high costs and a compromised reputation for the product’s developer.
This guide addresses how cell thawing has historically developed into the new techniques used today, along with the physical and biological implications of key metrics and components such as warming rate and ice structure. Also included are reviews of key studies from scientific literature and a consideration of the interactions between cooling and warming rates, as applicable to cell and gene therapies.
Thank you.You will receive an email shortly. Please check your inbox to download the Whitepaper.

Preview
Source: Pharmaceutical Technology
-->
By downloading this case study, you acknowledge that GlobalData may share your information with Cytiva Thematic and that your personal data will be used as described in their Privacy Policy
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Indications
Targets
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.





