The FDA's approval of Incyte’s Zynyz came as a surprise, its approval marks the eight checkpoint inhibitor to hit the market and the third indicated for metastatic MCC
27 Mar 2023
·
R&D
·Original
Drug ApprovalCell Therapy

Preview
Source: SYNAPSE
Mar. 22, 2023-- Incyte announced that the FDA has approved Zynyz(retifanlimab-dlwr), a humanized monoclonal antibody targeting programmed death receptor-1 (PD-1), for the treatment of adults with metastatic or recurrent locally advanced Merkel cell carcinoma (MCC). The FDA's approval of Incyte’s Zynyz came as a surprise, as the company hadn't disclosed an application filing or a PDUFA date. The drug got the go-ahead through the FDA’s accelerated approval pathway.
Zynyz's approval marks the eight
checkpoint inhibitor to hit the market
Zynyz®(Retifanlimab) is a programmed death receptor-1 (PD-1)-blocking antibody that works to treat Merkel cell carcinoma by binding to the PD-1 protein on immune T-cells to prevent the interaction with the PD-L1 protein on cancer cells. It removes the "brakes" on the immune system, allowing it to attack cancer cells more effectively.
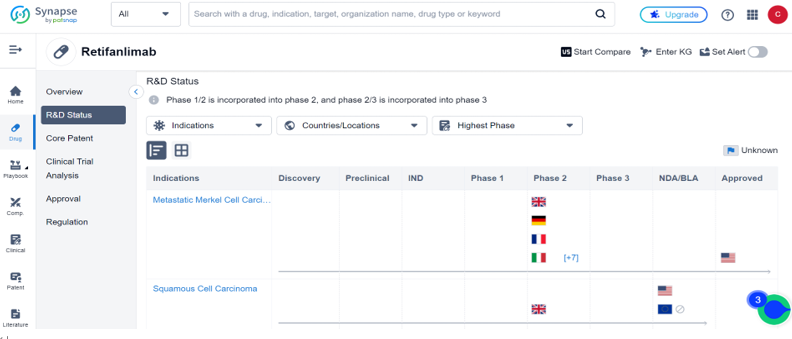
According to the drug Intelligence database: Synapse, there are currently 53 clinical trials for Retifanlimab worldwide, these trials are focused on 29 indications, including Metastatic Merkel Cell Carcinoma, Squamous Cell Carcinoma, Endometrial Carcinoma, and Non-Small Cell Lung Cancer. Metastatic Merkel Cell Carcinoma has been approved for sale in the United States, while other countries are in clinical Phase 2 development at most. Squamous Cell Carcinoma is in the BLA stage in the United States. For more detailed data, please refer to the Synapse.
Preview
Source: SYNAPSE
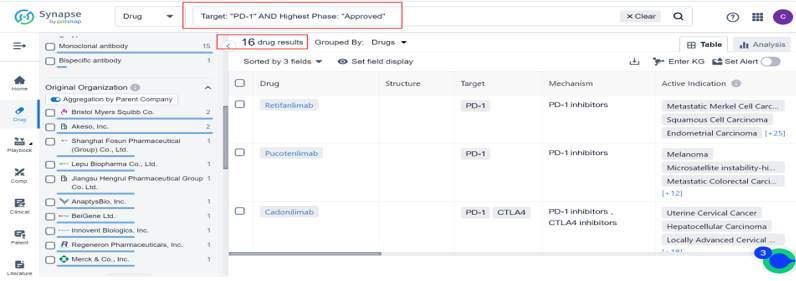
According to a search of the New Drug Intelligence database: Synapse, Zynyz is the 16th drug approved for PD-1, Merkel Cell Carcinoma is the 8th indication approved for PD-1, and the most approved indications were Hodgkin's Lymphom and Melanoma, followed by Metastatic Merkel Cell Carcinoma、Microsatellite instability-high cancer、Uterine Cervical Cancer、Microsatellite Instability cancer、Cutaneous Squamous Cell Carcinoma and Esophageal Carcinoma.
Preview
Source: SYNAPSE
The third indicated for metastatic
MCC, Zynyz
Merkel cell carcinoma (MCC) is a rare and aggressive type of skin cancer that frequently appears as a single, painless, reddish-purple skin nodule on the head, neck and arms in skin exposed to sunlight. MCC tends to grow quickly and has a high rate of metastatic disease, leading to a poor prognosis. The estimated five-year overall survival (OS) rate is 14% in patients with MCC who present with distant metastatic disease3. MCC
impacts less than 1 per 100,000 people in the U.S., but incidence rates are rapidly rising, especially in adults over the age of 65.
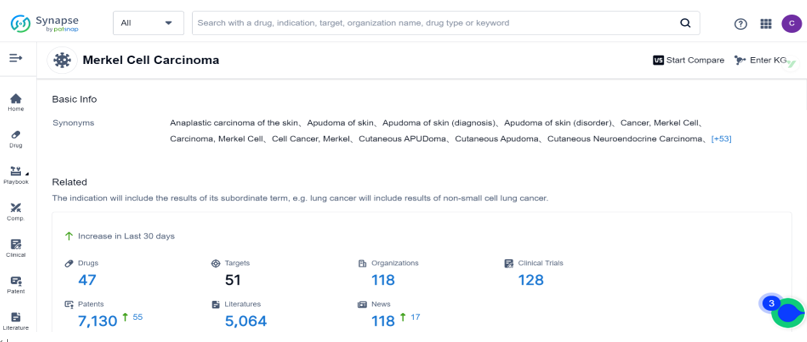
Search the drug intelligence database: Synapse, a total of 47 drugs and 128 clinical trials were found for the treatment of Merkel Cell Carcinoma. For this indication, the top three research and development organizations are Incyte Corp, Merck & Co., Inc. and Merck KGaA, the major R&D targets are focused on PD-1、PD-L1 and TLR9. The top three types of drug development for this indication are Monoclonal antibody, Small molecule drug, and Fusion protein.
Preview
Source: SYNAPSE
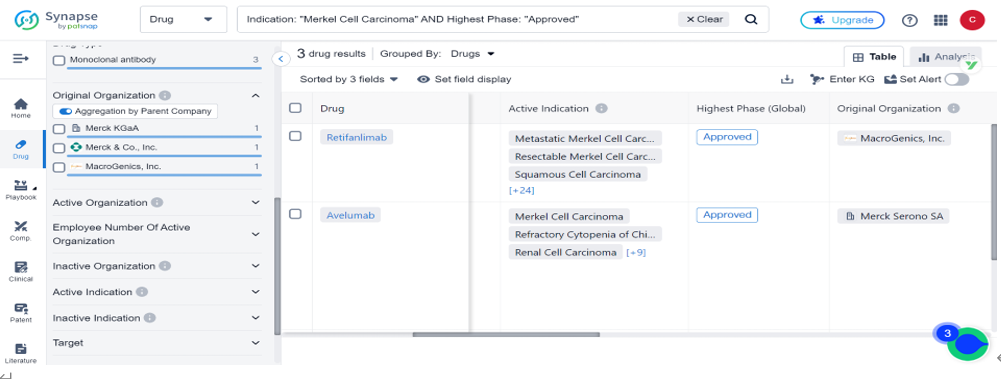
Zynyz is the third drug approved to be marketed for the treatment of Merkel cell carcinoma, the other two being Avelumab, approved in 2017, and Pembrolizumab, approved in 2018.
Preview
Source: SYNAPSE
“Zynyz offers patients and healthcare professionals an additional first-line anti-PD-1 option for patients with metastatic or recurrent locally advanced MCC, which can be a challenging and aggressive disease to treat,” said Hervé Hoppenot, Chief Executive Officer,
Incyte. “Incyte is grateful to the investigators and patients around the world who participated in the POD1UM-201 trial. We continue to study the potential of Zynyz in additional tumor types and in combination with other Incyte pipeline compounds.”
If you’re interested in learning more about this space or keeping track of drug development and clinical trials, sign up for Synapse ( synapse.patsnap.com ), our freemium product offering.
Organizations
Chat with Hiro
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.




