Merck admits defeat in race with Valneva to get first chikungunya vaccine to market
02 Feb 2023
Phase 3Phase 2Vaccine
Preview
Source: FierceBiotech
Merck confirmed to Fierce Biotech this morning that it has discontinued the chikungunya program, calling the decision part of a “routine pipeline prioritization.”
Once neck and neck with Valneva to get the first chikungunya vaccine to market, it now looks like Merck & Co. has admitted defeat as its French rival reaches the finish line.
Merck’s contender was V184, a vaccine acquired through the Big Pharma’s $366 million takeover of Themis in 2020. While the candidate completed a phase 2 trial, the study was suspended “due to a clinical stock recovery action” from December 2020 to the completion date in June 2021— meaning it never hit its original enrollment goal.
Merck confirmed to Fierce Biotech this morning that it has now discontinued the chikungunya program as part of a “routine pipeline prioritization.”
The sprint to approval was never just a two-horse race, however. Emergent BioSolutions is also fielding its own candidate, which is in two phase 3 trials. The biotech received an award from the U.S. Department of Defense last November to conduct further evaluations of the shot should it get approved.
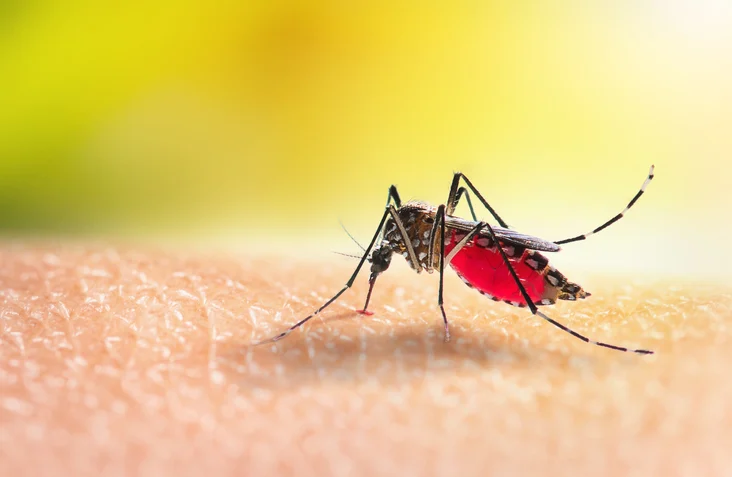
Chikungunya virus is spread to people by infected mosquitoes. Symptoms include fever, incapacitating joint pain, headache, muscle pain, joint swelling or rash. Like many tropical diseases, the reach of the virus is spreading into a wider range of countries.
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Indications
Targets
-Drugs
Chat with Hiro
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.




