Pie Medical Imaging announces 500th patient in the FAST III clinical trial
20 Jan 2023
Phase 3
MAASTRICHT, Netherlands, Jan. 20, 2023 /PRNewswire/ --
Pie Medical Imaging, a global leader in cardiac imaging,
announced the 500th patient inclusion in the FAST III, a multi-center randomized clinical trial, which investigates the
use of angiography-based vessel fractional flow reserve (CAAS vFFR) in patients undergoing coronary revascularization procedures.
In this trial, a CAAS vFFR guided strategy is compared to a FFR guided strategy to guide coronary revascularization.
The primary endpoint is a composite of all-cause death, any myocardial infarction, or any revascularization at 1-year post-randomization.
Continue Reading
Preview
Source: PRNewswire
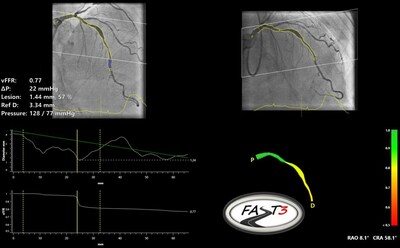
CAAS vFFR Software

Preview
Source: PRNewswire
Pie Medical Imaging Logo
The trial, led by Dr. Joost Daemen (cardiologist at the Thoraxcenter at the Erasmus University Medical Center, Rotterdam, The Netherlands),
is an investigator initiated international, multi-center randomized, non-inferiority trial.
500 patients are enrolled by hospitals with great expertise in coronary physiology at 25 sites across Europe in The Netherlands, Germany, Spain, Ireland, United Kingdom, Italy, and France. A total of
35 sites will be recruited with the final aim to include
2,228 patients by the end of the year.
CAAS vFFR (Pie Medical Imaging, Maastricht, The Netherlands) is an
angio-based FFR software suite to enable physiological assessment of intermediate coronary stenosis without the need of a pressure wire and adenosine.
The vFFR value can be calculated with solely two angiographic projections and the aortic root pressure. This vFFR has a
high correlation and diagnostic accuracy compared with wire based invasive FFR and NHPR measurements and the vFFR results are proven to be highly reproducible.
"The FAST III trial, together with several other studies currently being performed with CAAS vFFR, will increase the scientific body of evidence showing that the CAAS vFFR software is an excellent alternative to invasive wire-based physiological measurements," said
Rene Guillaume, Managing Director PMI.
The trial is funded by research grants from Pie Medical Imaging (Maastricht, the Netherlands) and Siemens Healthineers (Erlangen, Germany). The study is
sponsored by ECRI (European Cardiovascular Research Institute, Rotterdam-the Netherlands). Cardialysis (Rotterdam, The Netherlands) is responsible for trial services including trials management and Core Laboratory activities.
About Pie Medical Imaging
Pie Medical Imaging BV is a world leader in analysis and visualization of cardiovascular images. In Maastricht (The Netherlands), it hosts the global sales for the CAAS and 3mensio product lines. PMI and 3mensio Medical Imaging are part of the Esaote Group, leader in the biomedical equipment sector. More information about PMI is available at www.piemedicalimaging.com.
Photo - https://mma.prnewswire.com/media/1985989/Pie_Medical_Imaging.jpg
Logo - https://mma.prnewswire.com/media/1985988/Pie_Medical_Imaging_Logo.jpg
SOURCE Pie Medical Imaging (PMI)
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Indications
Targets
-Drugs
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.





