/ Not yet recruitingNot Applicable 单中心、随机、开放、双周期、双交叉设计评价中国健康受试者单次空腹及餐后口服双氯芬酸钠缓释片的人体生物等效性试验
[Translation] A single-center, randomized, open-label, two-period, double-crossover design study to evaluate the bioequivalence of diclofenac sodium sustained-release tablets in Chinese healthy subjects after single fasting and postprandial oral administration
主要目的:
以Novartis Pharma Schweiz AG生产的双氯芬酸钠缓释片(商品名:Voltaren® Retard,规格:100mg)为参比制剂,以四川华新制药有限公司生产的双氯芬酸钠缓释片(规格:0.1g)为受试制剂,通过单中心、随机、开放、双周期、双交叉的临床试验设计,对比在健康人体内空腹和餐后给药条件下的吸收速度和吸收程度,评价两种制剂的人体生物等效性。
次要目的:
评价受试制剂和参比制剂在中国健康受试者中的安全性。
[Translation] Main purpose:
The diclofenac sodium sustained-release tablets (trade name: Voltaren® Retard, specification: 100 mg) produced by Novartis Pharma Schweiz AG were used as the reference preparation, and the diclofenac sodium sustained-release tablets (specification: 0.1 g) produced by Sichuan Huaxin Pharmaceutical Co., Ltd. were used as the test preparation. Through a single-center, randomized, open, two-period, double-crossover clinical trial design, the absorption rate and degree of fasting and postprandial administration in healthy humans were compared to evaluate the bioequivalence of the two preparations.
Secondary purpose:
Evaluate the safety of the test preparation and the reference preparation in healthy Chinese subjects.
/ CompletedNot Applicable 单中心、随机、开放、双周期、双交叉设计评价中国健康受试者单次空腹及餐后口服双氯芬酸钠缓释片的人体生物等效性试验
[Translation] A single-center, randomized, open-label, two-period, double-crossover design study to evaluate the bioequivalence of diclofenac sodium sustained-release tablets in Chinese healthy subjects after single fasting and postprandial oral administration
主要目的:
以持证商为Novartis Pharma Schweiz AG的双氯芬酸钠缓释片(商品名:Voltaren®Retard,规格:100 mg)为参比制剂,以四川华新制药有限公司生产的双氯芬酸钠缓释片(规格:0.1 g)为受试制剂,通过单中心、随机、开放、双周期、双交叉的临床试验设计,对比在健康人体内空腹和餐后单次口服给药条件下的吸收速度和吸收程度,评价两种制剂的人体生物等效性。
次要目的:
观察及评价受试制剂和参比制剂在中国健康受试者中的安全性。
[Translation] Main purpose:
The diclofenac sodium sustained-release tablets (trade name: Voltaren® Retard, specification: 100 mg) produced by Novartis Pharma Schweiz AG were used as the reference preparation, and the diclofenac sodium sustained-release tablets (specification: 0.1 g) produced by Sichuan Huaxin Pharmaceutical Co., Ltd. were used as the test preparation. Through a single-center, randomized, open, double-period, double-crossover clinical trial design, the absorption rate and degree of single oral administration under fasting and postprandial conditions in healthy humans were compared to evaluate the bioequivalence of the two preparations in humans.
Secondary purpose:
Observe and evaluate the safety of the test preparation and the reference preparation in healthy Chinese subjects.
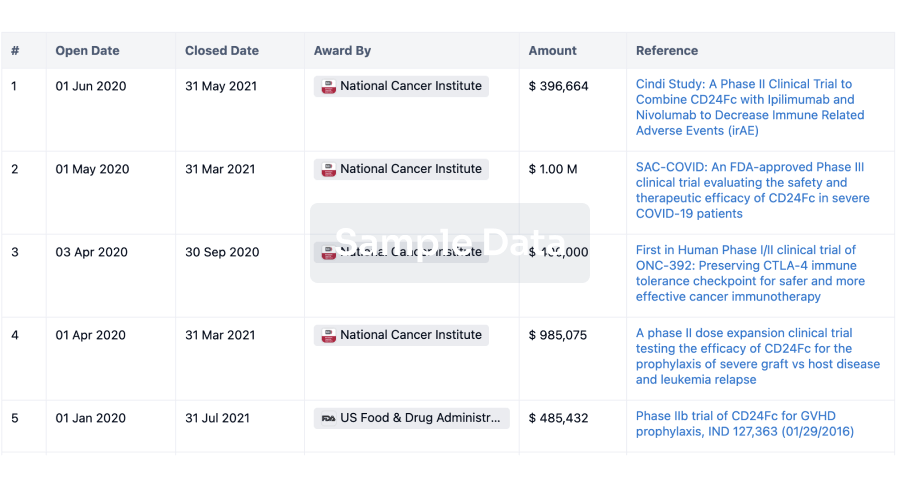
100 Clinical Results associated with Sichuan Huaxin Pharmaceutical Co., Ltd.
0 Patents (Medical) associated with Sichuan Huaxin Pharmaceutical Co., Ltd.
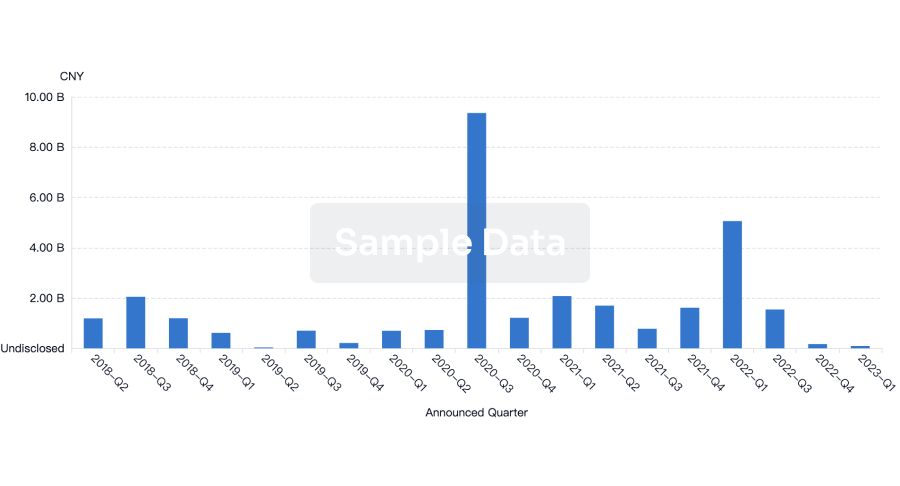
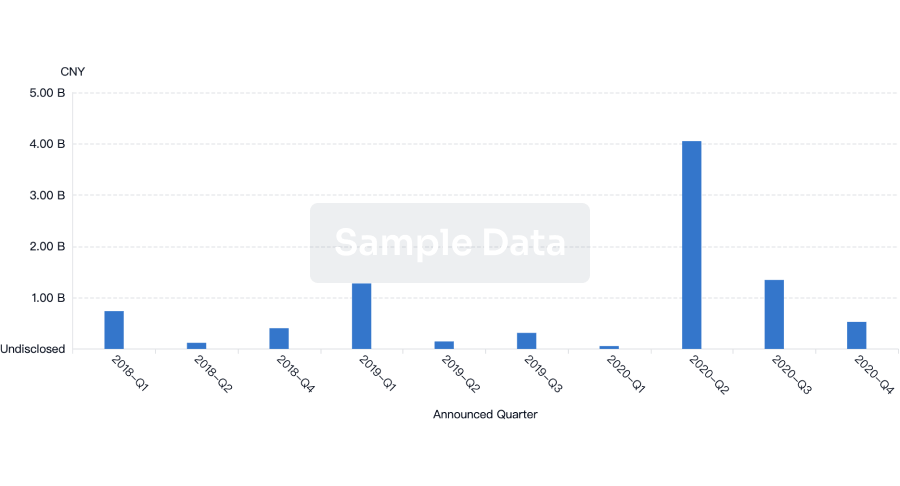
100 Deals associated with Sichuan Huaxin Pharmaceutical Co., Ltd.
100 Translational Medicine associated with Sichuan Huaxin Pharmaceutical Co., Ltd.