Request Demo
Last update 09 Sep 2025

GemVax & KAEL Co., Ltd.
Last update 09 Sep 2025
Overview
Tags
Nervous System Diseases
Other Diseases
Endocrinology and Metabolic Disease
Synthetic peptide vaccine
Therapeutic vaccine
Disease domain score
A glimpse into the focused therapeutic areas
No Data
Technology Platform
Most used technologies in drug development
No Data
Targets
Most frequently developed targets
No Data
| Disease Domain | Count |
|---|---|
| Neoplasms | 3 |
| Nervous System Diseases | 1 |
| Immune System Diseases | 1 |
| Endocrinology and Metabolic Disease | 1 |
| Top 5 Drug Type | Count |
|---|---|
| Therapeutic vaccine | 2 |
| Unknown | 1 |
| Synthetic peptide | 1 |
| Synthetic peptide vaccine | 1 |
| Top 5 Target | Count |
|---|---|
| TERT(Telomerase reverse transcriptase) | 1 |
| Mitochondrial proteins | 1 |
Related
4
Drugs associated with GemVax & KAEL Co., Ltd.Target |
Mechanism TERT inhibitors |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication |
Drug Highest PhaseApproved |
First Approval Ctry. / Loc. South Korea |
First Approval Date15 Sep 2015 |
Target- |
Mechanism Immunostimulants |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication- |
Drug Highest PhasePhase 2 |
First Approval Ctry. / Loc.- |
First Approval Date- |
Target |
Mechanism Mitochondrial proteins inhibitors |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication |
Drug Highest PhasePhase 2 |
First Approval Ctry. / Loc.- |
First Approval Date- |
13
Clinical Trials associated with GemVax & KAEL Co., Ltd.NCT06625710
A Randomized, Double-blind, Placebo Controlled, Dose-escalation Phase 1 Clinical Trial to Evaluate the Safety, Tolerability and Pharmacokinetic Characteristics After Single and Multiple Administration of GV1001 in Healthy Subjects
The goal of this clinical trial is to assess the the safety, tolerability and pharmacokinetic characteristics after single and multiple administration of GV1001 in healthy subjects. The main questions it aims to answer are:
Safety and Tolerability of GV1001 in different dose scheme, and PK Characteristics of GV1001 in differenct dose scheme
Part A: single dose, 8 days clinical trial participation including one 3-day hospitalization
Part B & Extra Cohort: Multiple dose, 42 days clinical trial participation (12 days treatment + 30 days safety follow-up) including two times of 3-day hospitalization.
Safety and Tolerability of GV1001 in different dose scheme, and PK Characteristics of GV1001 in differenct dose scheme
Part A: single dose, 8 days clinical trial participation including one 3-day hospitalization
Part B & Extra Cohort: Multiple dose, 42 days clinical trial participation (12 days treatment + 30 days safety follow-up) including two times of 3-day hospitalization.
Start Date08 Oct 2024 |
Sponsor / Collaborator |
NCT06235775
Twelve-months Extension Study to Explore the Long-Term Safety and Efficacy of Subcutaneous Administration of GV1001 1.12 Mg/day in Patients with Progressive Supranuclear Palsy Who Completed Study GV1001-PSP-CL2-011
The study will be conducted by the Sponsor to evaluate Twelve-months Long-Term Safety and Efficacy of GV1001 (1.12 mg) administered subcutaneously as a treatment for Progressive Supranuclear Palsy(PSP). In 75 patients diagnosed with PSP Richardson(PSP-RS) or PSP-Parkinsonism (PSP-P) who Completed Study GV1001-PSP-CL2-011.
Start Date12 Dec 2023 |
Sponsor / Collaborator |
NCT05819658
A Multi-center, Randomized, Double-blind, Placebo-controlled, Parallel Design, Prospective, Phase IIa Exploratory Clinical Trial to Evaluate the Efficacy and Safety of SC Administration of GV1001 0.56 or 1.12 Mg/day in Patients with PSP
The study will be conducted by the Sponsor to evaluate the efficacy and safety of GV1001 (0.56 mg and 1.12 mg) administered subcutaneously as a treatment for Progressive Supranuclear Palsy, (PSP). In 75 patients diagnosed with PSPR Richardson(PSP-RS) or PSP-Parkinsonism (PSP-P) at five hospitals in Korea, subcutaneous administration of GV1001 0.56 or 1.12 mg/day will be conducted with multicenter, randomized, double-blind, placebo-controlled, parallel design, prospective phase 2a.
Start Date14 Jun 2023 |
Sponsor / Collaborator |
100 Clinical Results associated with GemVax & KAEL Co., Ltd.
Login to view more data
0 Patents (Medical) associated with GemVax & KAEL Co., Ltd.
Login to view more data
20
Literatures (Medical) associated with GemVax & KAEL Co., Ltd.15 Apr 2022·Aging-USQ3 · MEDICINE
Association between sleep parameters and longitudinal shortening of telomere length
Q3 · MEDICINE
Article
Author: Park, Hyun-Hee ; Park, Kyung Won ; Yoon, Soo Jin ; Choi, Seong Hye ; Hong, Jin Yong ; Kwon, Hyuk Sung ; Ha, Jungsoon ; Kim, Eun-Joo ; Han, Myung Hoon ; Lee, Eun-Hye ; Kim, Hee Jin ; Jin, Jeong-Hwa ; Park, Jong Eun ; Koh, Seong-Ho ; Jang, Jae-Won ; Jeong, Jee Hyang ; Yoon, Bora
BACKGROUND:
The relationship between sleep parameters and longitudinal shortening of telomere length is unclear. This study aimed to investigate the relationship between sleep parameters and the shortening of leukocyte telomere length (LTL) over a year.
METHODS:
Among the participants in the validation cohort of the Korea Brain Aging Study for the Early Diagnosis and Prediction of Alzheimer's Disease, participants who measured both baseline and follow-up (two years later) of LTL were analyzed. They were dichotomized according to the degree of LTL attrition over two years. Clinical characteristics were compared between the faster and slower LTL shortening groups (cut-off points: -0.710 kbp, n = 119 each). Multivariable logistic regression analyses were performed to determine independent relationships between faster shortening of LTL length and sleep parameters.
RESULTS:
A total of 238 participants, aged 55-88 years, were included. Participants with faster LTL shortening had a shorter duration of sleep (P = 0.013) and longer sleep latency (P = 0.007). Among the components of the PSQI, subjective measures of sleep quality, sleep latency, sleep duration, and sleep efficiency were significantly worse in participants with faster LTL shortening. Multivariate logistic regression analysis showed that sleep duration (per hour, OR = 0.831, 95% CI = 0.698-0.989), sleep latency (per minute, OR = 1.013, 95% CI = 1.002-1.024), global PSQI score (OR = 1.134, 95% CI = 1.040-1.236), shortest sleep duration (OR = 5.173, 95% CI = 1.563-17.126), and lowest sleep efficiency (OR = 7.351, 95% CI = 1.943-27.946) were independently associated with faster LTL shortening.
CONCLUSIONS:
Poor sleep quality, specifically short sleep duration, long sleep latency, and low sleep efficiency were associated with faster longitudinal shortening of LTL.
01 Sep 2021·Journal of the neurological sciencesQ4 · MEDICINE
Increased telomere length in patients with frontotemporal dementia syndrome
Q4 · MEDICINE
Article
Author: Yoon, Soo Jin ; Na, Duk L ; Kwon, Hyuk Sung ; Roh, Jee Hoon ; Kim, Hee-Jin ; Kim, Byeong C ; Park, Hyun-Hee ; Jang, Jae-Won ; Park, Young Ho ; Kwon, Jay C ; Jeong, Jee H ; Kim, SangYun ; Kim, Young-Eun ; Kim, Eun-Joo ; Park, Kee Hyung ; Ha, Jungsoon ; Choi, Seong Hye ; Lee, Jae-Hong ; Jin, Jeong-Hwa ; Seo, Sang Won ; Jung, Na-Yeon ; Park, Kyung Won ; Koh, Seong-Ho
BACKGROUND:
Telomeres are repetitive DNA sequences of TTAGGG at the ends of chromosomes. Many studies have shown that telomere shortening is associated with aging-related diseases, such as cardiovascular diseases, hypertension, diabetes, cancer, and various neurodegenerative diseases, including Alzheimer's disease, vascular dementia, Parkinson's disease, and dementia with Lewy bodies. However, changes in telomere length (TL) in patients with frontotemporal dementia (FTD) syndrome are unclear. Accordingly, in this study, we assessed TL in blood samples from patients with FTD syndrome.
METHODS:
Absolute TL was measured in peripheral blood leukocytes from 53 patients with FTD syndromes (25 with behavioral variant FTD, 19 with semantic variant primary progressive aphasia [PPA], six with nonfluent/agrammatic variant PPA, and three with amyotrophic lateral sclerosis [ALS] plus) and 28 cognitively unimpaired (CU) controls using terminal restriction fragment analysis.
RESULTS:
TL was significantly longer in the FTD group than in the CU group. All FTD subtypes had significantly longer TL than controls. There were no significant differences in TL among FTD syndromes. No significant correlations were found between TL and demographic factors in the FTD group.
CONCLUSIONS:
Longer telomeres were associated with FTD syndrome, consistent with a recent report demonstrating that longer telomeres are related to ALS. Therefore, our results may support a shared biology between FTD and ALS. More studies with larger sample sizes are needed.
01 Apr 2021·Molecular neurobiologyQ2 · MEDICINE
Telmisartan Inhibits the NLRP3 Inflammasome by Activating the PI3K Pathway in Neural Stem Cells Injured by Oxygen-Glucose Deprivation
Q2 · MEDICINE
Article
Author: Koh, Seong-Ho ; Kwon, Hyuk Sung ; Lee, Kyu-Yong ; Choi, Hojin ; Ha, Jungsoon ; Park, Hyun-Hee ; Kim, Ji Young ; Lee, Young Joo ; Lee, Eun-Hye
Angiotensin II receptor blockers (ARBs) have been shown to exert neuroprotective effects by suppressing inflammatory and apoptotic responses. In the present study, the effects of the ARB telmisartan on the NLRP3 inflammasome induced by oxygen-glucose deprivation (OGD) in neural stem cells (NSCs) were investigated, as well as their possible association with the activation of the PI3K pathway. Cultured NSCs were treated with different concentrations of telmisartan and subjected to various durations of OGD. Cell counting, lactate dehydrogenase, bromodeoxyuridine, and colony-forming unit assays were performed to measure cell viability and proliferation. In addition, the activity of intracellular signaling pathways associated with the PI3K pathway and NLRP3 inflammasome was evaluated. Telmisartan alone did not affect NSCs up to a concentration of 10 μM under normal conditions but showed toxicity at a concentration of 100 μM. Moreover, OGD reduced the viability of NSCs in a time-dependent manner. Nevertheless, treatment with telmisartan increased the viability and proliferation of OGD-injured NSCs. Furthermore, telmisartan promoted the expression of survival-related proteins and mRNA while inhibiting the expression of death-related proteins induced by OGD. In particular, telmisartan attenuated OGD-dependent expression of the NLRP3 inflammasome and its related signaling proteins. These beneficial effects of telmisartan were blocked by a PI3K inhibitor. Together, these results indicate that telmisartan attenuated the activation of the NLRP3 inflammasome by triggering the PI3K pathway, thereby contributing to neuroprotection.
5
News (Medical) associated with GemVax & KAEL Co., Ltd.30 Oct 2024
–
Topline supports moving to Phase 3 trial and shows potential to develop GV1001 as the world’s first PSP treatment
SEOUL, South Korea I October 29, 2024 I
GemVax & KAEL Co., Ltd. (“GemVax”; KOSDAQ ticker: 082270) announced that topline results of a Phase 2a clinical trial (the “Phase 2a PSP Clinical Trial”) of GV1001, an investigational peptide drug for the treatment of progressive supranuclear palsy (“PSP”), were presented at “Neuro2024: The PSP and CBD International Research Symposium” in Toronto, Canada, at 4:45 p.m. local time on 24
th
October.
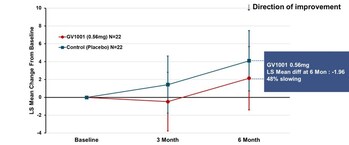
Figure 1. PSP-Rating Scale Total Score (PSP-RS Type + PSP-P Type_ LS mean using MMRM) (PRNewsfoto/GemVax & KAEL)
Figure 2. PSP-Rating Scale Total Score (PSP-RS Type_ simple average) (PRNewsfoto/GemVax & KAEL)
Figure 3. Responder Rate (PSP-RS Type) (PRNewsfoto/GemVax & KAEL)
PSP is a degenerative disease that, like Parkinson’s disease, causes symptoms such as gait disturbances, early falls, vertical gaze palsy, rigidity, tremors, and cognitive decline, but it progresses faster and currently has no fundamental treatment. PSP is classified into several types, including PSP-Richardson’s syndrome (“PSP-RS”) and PSP-parkinsonism (“PSP-P”). Compared to other types of PSP, the PSP-RS type shows a greater accumulation of tau protein and affects larger areas, including the cerebellum, dentate nucleus, pontine nuclei, frontal lobe, and parietal lobe.
The Phase 2a PSP Clinical Trial was a 24-week, randomized, double-blind, placebo-controlled, prospective exploratory clinical trial conducted in 78 patients with PSP at 5 centers in Korea. The participants were randomized 1:1:1 to receive either placebo or GV1001 0.56 mg or GV1001 1.12 mg administered subcutaneously once weekly for the first 4 weeks (1 month), and then at 2-week intervals for 20 weeks (5 months) for a total of 24 weeks (6 months). Patients with both PSP-RS and PSP-P types were eligible to participate in the study. Results showed higher benefits in the lower dose group (0.56 mg), particularly in PSP-RS type patients.
The primary endpoint of the trial was change from baseline in total score (calculated as the least-square mean using MMRM method) of PSP-Rating Scale after 24 weeks of GV1001 administration, which showed deterioration by 2.14 points in GV1001 0.56 mg dose group compared to 4.10 points in the placebo group, demonstrating a 48% reduction in disease progression (see Figure 1). Although statistical significance was not demonstrated, the results support the potential of GV1001 as a treatment of PSP, a disease for which there is currently no cure, and the potential to advance GV1001 into further clinical trials.
The clinically typical PSP is often referred to as the PSP-RS type, which accounts for the majority of PSP patients. This type progresses faster and has a shorter average survival time compared to other PSP types. Subgroup analysis was conducted in patients with PSP-RS type only. The change from baseline in PSP-Rating Scale total score mean (calculated using simple average) at 24 weeks of GV1001 administration to PSP-RS type patients was a deterioration by 0.25 points in the GV1001 0.56 mg dose group compared to a deterioration by 5.19 points in the placebo group, demonstrating a 4.94-point difference or a 95% reduction in disease progression (see Figure 2).
Many PSP-RS type patients in the treatment group experienced symptom stabilization or even improvement during the clinical period. When calculated as responder rate based on the percentage of patients whose PSP Rating Scale scores improved or remained stable after six months of treatment compared to baseline, 58.33% of PSP-RS type patients in the 0.56 mg GV1001 group showed improvement or stabilization (see Figure3).
The safety profile of GV1001 in the Phase 2a PSP Clinical Trial was consistent with prior safety data. GV1001 was generally well-tolerated with no serious adverse events related to the drug reported.
Hyungsik Moon, CSO of GemVax, stated that “this Phase 2a trial was an exploratory study to determine the optimal dosage and find out how the peptide works on different subgroups. Although the topline result did not achieve statistical significance, the evidence is strong enough to consider moving forward to a pivotal trial and shows potential to develop GV1001 as the world’s first treatment option for PSP.”
Experts at the Neuro2024 meeting welcomed the results of the PSP trial as encouraging and expressed excitement for the drug to enter a global Phase 3 clinical trial for further development.
“This pilot study was not fully powered and the treatment duration with 6 months was short. Thus, statistically significant confirmatory results could not be expected” said Peter Schüler, MD, Senior Vice President of Drug Development at global CRO ICON. “Nonetheless, the observed trends are very plausible and consistent in two domains: motor performance and cognitive function, both favoring the lower dose group.”
“The trial identified the optimal dose, which was one of the primary objectives of the Phase 2a study, and demonstrated clinically meaningful benefits, namely full stabilization of the disease compared to the placebo group,” said Dr. Schüler, adding “these topline results provide a strong foundation for advancing to Phase 3.”
Dr. Günter U. Höglinger, Head of the Department of Neurology, LMU Hospital, Munich, and a world-renowned expert in PSP, commented: “very exciting Phase 2 level data with novel drug study with new mechanisms of action. Data is preliminary but very promising and it is in line with [GV1001] Alzheimer’s disease clinical data. I look forward to further development and very excited to participate and lead the [PSP] Phase 3 study.”
Dr. Kristophe Diaz, Director of CurePSP, said that “we are encouraged by the results of the recent GemVax clinical trial, which offer hope to the entire PSP community, including patients who currently have no treatment options, their families and the physicians who care for them” and that “we congratulate GemVax on the successful completion of this trial and look forward to further developments that benefit the PSP community.” He also said that “CurePSP remains committed to collaborating and supporting efforts that bring hope and progress for those affected by this devastating disease.”
About Phase 2a PSP Clinical Trial (NCT05819658)
The Phase 2a PSP clinical trial was a 24-week, multicenter, randomized, double-blind, placebo-controlled, prospective phase 2a exploratory clinical trial to evaluate the safety and efficacy of GV1001 0.56 mg or 1.12 mg compared to placebo for the treatment of patients with PSP. The primary outcome of the study was change from baseline in the total score of PSP-Rating Scale after 24 weeks of GV1001 administration. Secondary endpoints included change from baseline in the total score of PSP-Rating Scale at 3 months, MoCA-K, K-FAB and ES-ADL at both 3 and 6 months. Overall safety of GV1001 administration was also assessed.
About GV1001
GV1001 is a synthetic peptide consisting of 16 amino acids based on the key sequence of telomerase. GV1001 has been studied for the potential treatment of neurodegenerative diseases including Alzheimer’s disease and PSP. In neurodegenerative diseases, GV1001 has been demonstrated to modulate phenotypes of glial cells, and to regulate neuroinflammation. In addition to the Phase 2a PSP clinical trial, a Phase 2 Alzheimer’s disease clinical trial of GV1001 is currently ongoing in the U.S. and Europe (NCT05189210).
About PSP
Progressive supranuclear palsy is a rare progressive and adult-onset neurodegenerative disease that currently has no disease-modifying drug. Approximately seven in 100,000 people worldwide is affected by PSP and is more common in men. People over the age of 60 are mainly affected. The symptoms of PSP include loss of balance, changes in personality, weakness of eye movements, especially in the downward direction, difficulty in swallowing, slurred speech and cognitive impairment.
About GemVax & KAEL
GemVax & KAEL Co., Ltd. is a pioneering clinical-stage biopharmaceutical company based in Korea, dedicated to developing proprietary therapeutics for neurodegenerative diseases including progressive supranuclear palsy and Alzheimer’s disease. As for PSP, GemVax is currently conducting a Phase 2a study in Korea to evaluate the efficacy and safety of GV1001 in patients with PSP. Preparations are also underway for a global PSP clinical trial. In addition, GemVax is currently conducting a Phase 2 Alzheimer’s disease clinical trial in the U.S. and Europe. For more information, visit
www.gemvax.com
and follow us on
Linkedin
.
SOURCE:
GemVax
Phase 2Clinical Result
29 Oct 2024
- Topline supports moving to Phase 3 trial and shows potential to develop GV1001 as the world's first PSP treatment
SEOUL, South Korea, Oct. 29, 2024 /PRNewswire/ -- GemVax & KAEL Co., Ltd. ("GemVax"; KOSDAQ ticker: 082270) announced that topline results of a Phase 2a clinical trial (the "Phase 2a PSP Clinical Trial") of GV1001, an investigational peptide drug for the treatment of progressive supranuclear palsy ("PSP"), were presented at "Neuro2024: The PSP and CBD International Research Symposium" in Toronto, Canada, at 4:45 p.m. local time on 24th October.
Continue Reading
Figure 1. PSP-Rating Scale Total Score (PSP-RS Type + PSP-P Type_ LS mean using MMRM) (PRNewsfoto/GemVax & KAEL)
Figure 2. PSP-Rating Scale Total Score (PSP-RS Type_ simple average) (PRNewsfoto/GemVax & KAEL)
Figure 3. Responder Rate (PSP-RS Type) (PRNewsfoto/GemVax & KAEL)
View PDF
Phase 2PDCPhase 3
19 Sep 2022
Researchers found that when levels of an RNA molecule called TERRA levels were overexpressed, treatment-resistant ALT cancer cells stopped dividing and died.
Alternative lengthening of telomeres cells, or ALT cells, have long presented a challenge for researchers and oncologists alike. Unlike other cancer cells, which use the enzyme telomerase to lengthen their telomeres and grow indefinitely, ALT cells don’t possess telomerase. Instead, these treatment-resistant cells have other means of keeping their telomeres from degrading.
Now, a team of researchers from Portugal’s Instituto de Medicina Molecular João Lobo Antunes reported Monday that it has found the RNA molecule involved in preserving telomere length in ALT cells could perhaps be used against them.
The molecule, called telomeric repeat-containing RNA, or TERRA, is present in low levels in normal cells but is far more abundant in ALT cells. There, it causes controlled DNA damage that is then repaired in a process called break-induced telomere synthesis, or BITS, ultimately increasing the telomeres’ length. But it appears there is a limit to how much damage ALT-cell telomeres can handle: The research team found that when TERRA levels were expressed beyond a certain level, the cells would stop dividing and die.
“When the levels of TERRA are increased, the damage at telomeres also increases and this becomes so heavy that even a cancer cell that is usually more resistant is not able to multiply anymore,” first author Bruno Silva, Ph.D., a senior postdoctoral researcher at the institute, said in a release.
The study builds upon the team’s previous work on TERRA, where they developed a first-of-its-kind molecule to inhibit its transcription and ultimately reduced DNA damage to ALT cell telomeres, impairing their elongation.
In their newest study—published in the Proceedings of the National Academy of Sciences—the researchers looked at what would happen if they did the opposite, using molecular tools to overexpress TERRA in ALT cells. They hypothesized that there might be a threshold beyond which damage to a cell’s telomeres would be too much for repair mechanisms to keep up with.
This turned out to be the case: High levels of damage by TERRA overwhelmed the usual DNA repair mechanisms so much that the cells needed to recruit other telomeres to help. This led to the loss of multiple telomeres at once and stunted the cells’ division.
Together, the results of the researchers’ previous work and their new study suggest that TERRA might be a “uniquely versatile” therapeutic target, Silva said.
“By decreasing its levels we can block telomere maintenance, as we have shown before, while by increasing TERRA levels, we can rise the damage to levels that are not sustainable even for a cancer cell, eventually leading to cell death,” he said.
The team is currently in the process of identifying the proteins that activate or inhibit the expression of TERRA. From there, they plan to develop small molecules targeting those factors and test them out against ALT cancer cells, Silva told Fierce Biotech in an email.
While a number of telomerase inhibitors have been developed to treat various types of cancer—such as Geron’s imetelstat, Invectys’ cancer vaccine INVAC-1 and riavax, another cancer vaccine from Korean firm GemVax & Kael—drugs targeting TERRA have not yet been developed.
VaccineSmall molecular drug
100 Deals associated with GemVax & KAEL Co., Ltd.
Login to view more data
100 Translational Medicine associated with GemVax & KAEL Co., Ltd.
Login to view more data
Corporation Tree
Boost your research with our corporation tree data.
login
or

Pipeline
Pipeline Snapshot as of 22 Feb 2026
The statistics for drugs in the Pipeline is the current organization and its subsidiaries are counted as organizations,Early Phase 1 is incorporated into Phase 1, Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3
Preclinical
1
2
Phase 2
Approved
1
9
Other
Login to view more data
Current Projects
| Drug(Targets) | Indications | Global Highest Phase |
|---|---|---|
Tertomotide hydrochloride ( TERT ) | Alzheimer Disease More | Phase 2 |
MSI-vaccine(KAEL-GemVax) | Colorectal Cancer More | Phase 2 |
HIV-1 CTL epitope-based DNA vaccine(VaxOnco, Inc.) | HIV Infections More | Discontinued |
HIV vaccines polytope(KAEL-GemVax Co., Ltd.) | HIV Infections More | Discontinued |
PX-1041 ( EGF ) | Breast Cancer More | Discontinued |
Login to view more data
Deal
Boost your decision using our deal data.
login
or

Translational Medicine
Boost your research with our translational medicine data.
login
or

Profit
Explore the financial positions of over 360K organizations with Synapse.
login
or

Grant & Funding(NIH)
Access more than 2 million grant and funding information to elevate your research journey.
login
or

Investment
Gain insights on the latest company investments from start-ups to established corporations.
login
or

Financing
Unearth financing trends to validate and advance investment opportunities.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
